
Click to explore the KEYNOTE-522 overall survival results (secondary efficacy outcome measure)
Unmet need
In general, some patients with TNBC have poor outcomes due to recurrence despite their initial treatments.2-4
These high recurrence rates highlight the need for more effective treatments.3
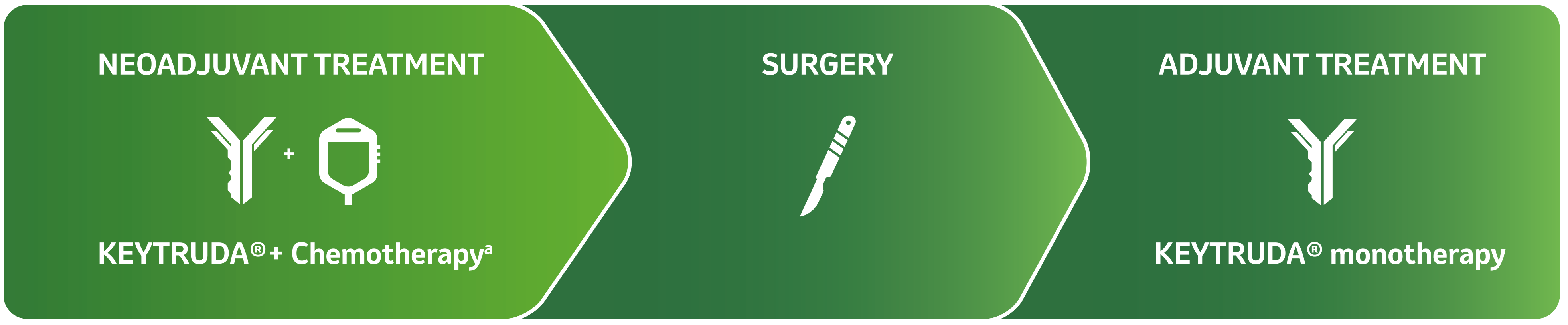
KEYTRUDA® (pembrolizumab), in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advanced, or early-stage triple-negative breast cancer (TNBC) at high risk of recurrence.5

The first and only anti–PD-1 indicated for high-risk early-stage TNBC as a neoadjuvant combination treatment followed by adjuvant monotherapy treatment.5-10

Discover KEYNOTE-522: Efficacy insights
KEYNOTE-522 evaluates the efficacy and safety of neoadjuvant KEYTRUDA® + chemotherapya followed by adjuvant KEYTRUDA® in eTNBC.5
aChemotherapy: Carboplatin and paclitaxel followed by (doxorubicin or epirubicin) and cyclophosphamide.
eTNBC: early triple-negative breast cancer; PD-L1: programmed death ligand 1; TNBC: triple-negative breast cancer.
Watch Professor Schmid, lead investigator of the KEYNOTE-522 trial, discuss the latest results
“The KEYNOTE-522 trial has been a practice-changing trial for patients with stage II or stage III early triple-negative breast cancer.”
Prof. Peter Schmid, FRCP, MD, PhD
Professor Schmid is the clinical director of the Breast Cancer Centre and an honorary consultant medical oncologist at St. Bartholomew’s Hospital, London. He is also the Chair in Cancer Medicine and the Lead of the Centre of Experimental Cancer Medicine at Barts Cancer Institute, Queen Mary University London. Professor Schmid is the lead investigator of the KEYNOTE-522 trial in eTNBC.
eTNBC: early triple-negative breast cancer; FRCP: Fellowship of Royal College of Physicians.
KEYNOTE-522 is a phase III, randomised, multicentre, double-blind, placebo-controlled trial that enrolled 1,174 patients with high-risk, locally advanced or eTNBC.
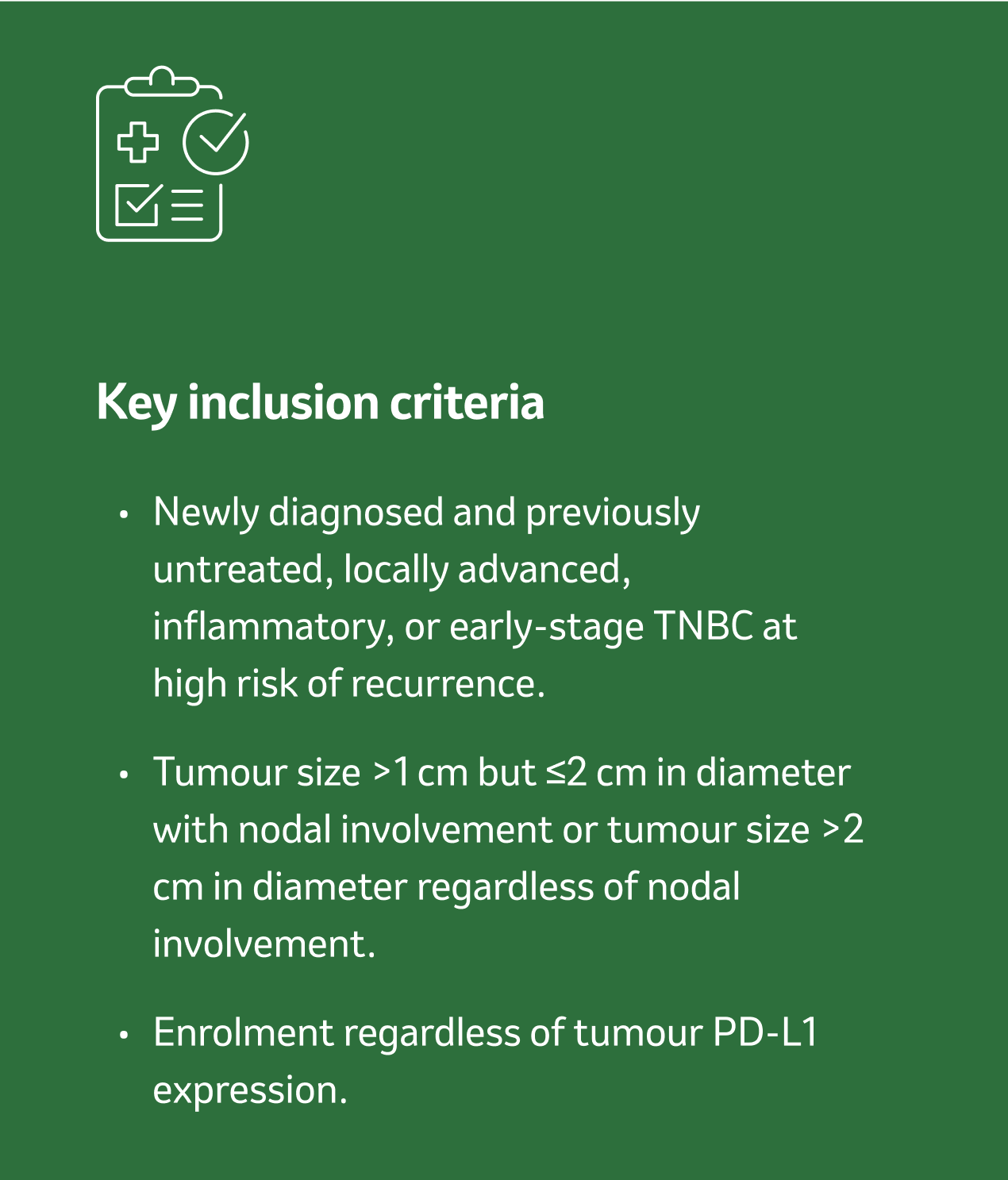

For additional study eligibility criteria, see Schmid P et al., 2020.11
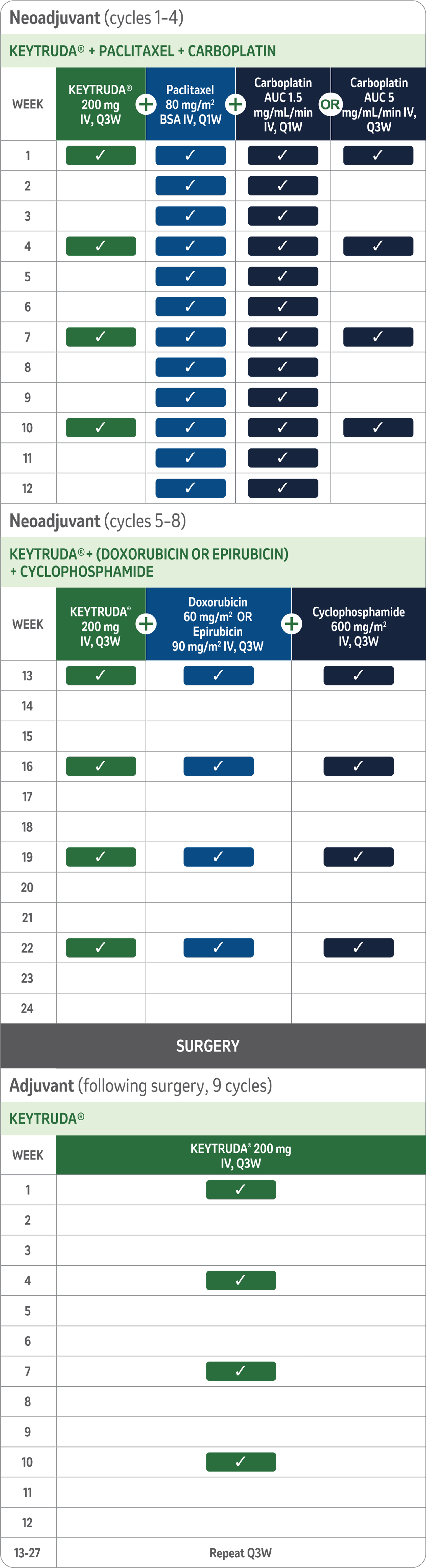
Study treatment arms5,11
- 2:1 ratio randomisation to the following treatment arms.b,c
- All study medications were administered via intravenous infusion.
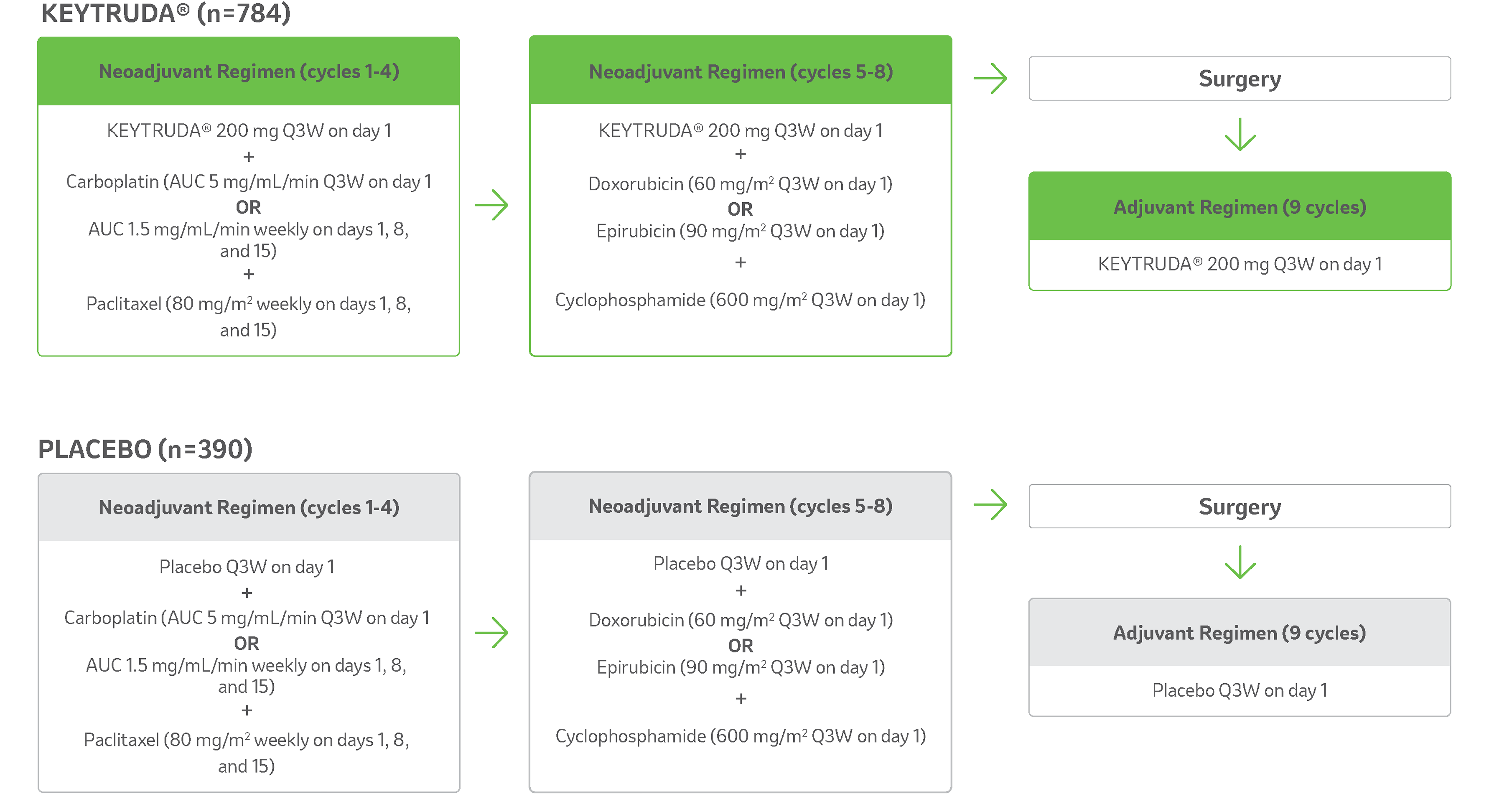
- Treatment with KEYTRUDA® or placebo continued until completion of treatment (17 cycles), disease progression that precludes definitive surgery, disease recurrence in the adjuvant phase, or unacceptable toxicity.
Primary efficacy outcome measures
- pCR: Absence of invasive cancer in the breast and lymph nodes (ypT0/Tis ypN0) and was assessed by the blinded local pathologist at the time of definitive surgery.5
- EFS: The time from randomisation to the first occurrence of any of the following events: progression of disease that precludes definitive surgery, local or distant recurrence, second primary malignancy, or death due to any cause, in the intention-to-treat population.5
Secondary efficacy outcome measures
- OS defined as the time from randomisation to death from any cause (key secondary efficacy outcome measure).1
- pCR defined as ypT0 ypN0 and ypT0/Tis in all patients.11
- pCR according to all definitions in patients with PD-L1–positive tumours.11
- EFS among patients with PD-L1–positive tumours.11
Safety measures
- Safety during the neoadjuvant and adjuvant phases was evaluated in all patients who received at least one trial drug, underwent surgery, or both.11
bKEYNOTE-522 is a phase III, randomised, multicentre, double-blind, placebo-controlled trial that enrolled 1,174 patients with newly diagnosed, previously untreated high-risk, locally advanced early-stage or TNBC. Patients were randomised 2:1 to receive either KEYTRUDA® in combination with chemotherapya as neoadjuvant treatment followed by KEYTRUDA® monotherapy as adjuvant treatment after surgery, or placebo and chemotherapya as neoadjuvant treatment followed by placebo after surgery. Treatment with KEYTRUDA® or placebo continued until completion of treatment (17 cycles), disease progression that precludes definitive surgery, disease recurrence in the adjuvant phase, or unacceptable toxicity. The primary efficacy outcome measures were pathological complete response and event-free survival. Overall survival was one of several secondary efficacy outcome measures.
cRandomisation was stratified by nodal status (positive vs negative), tumour size (T1/T2 vs T3/T4), and choice of carboplatin (dosed every 3 weeks vs weekly).
AUC: area under the curve; EFS: event-free survival; eTNBC: early triple-negative breast cancer; N: nodal involvement; OS: overall survival; pCR: pathological complete response; PD-L1: programmed death ligand 1; Q3W: every 3 weeks.
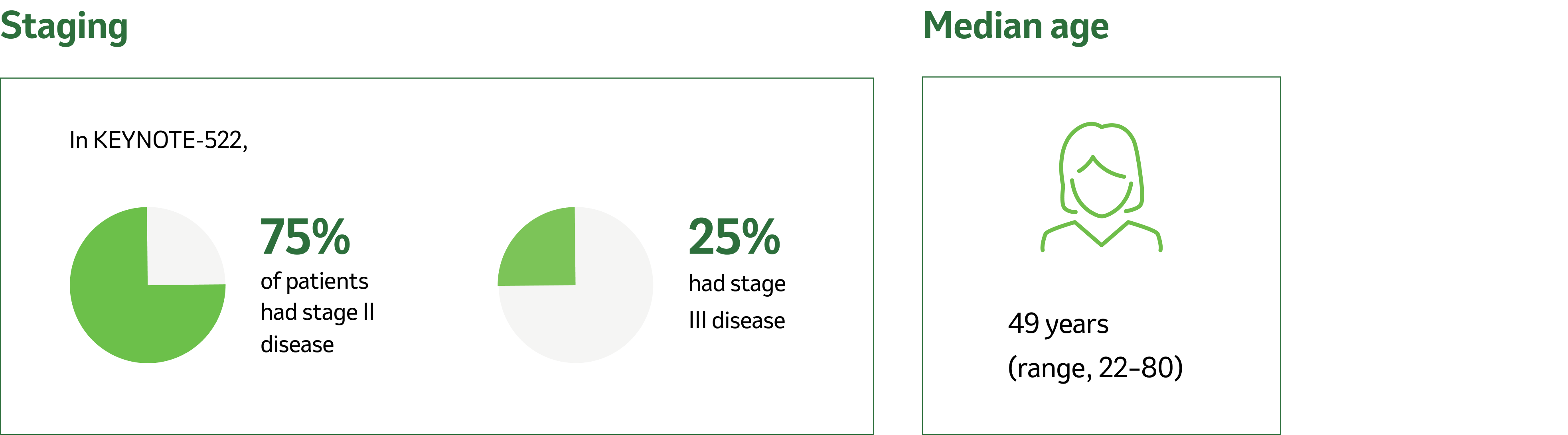
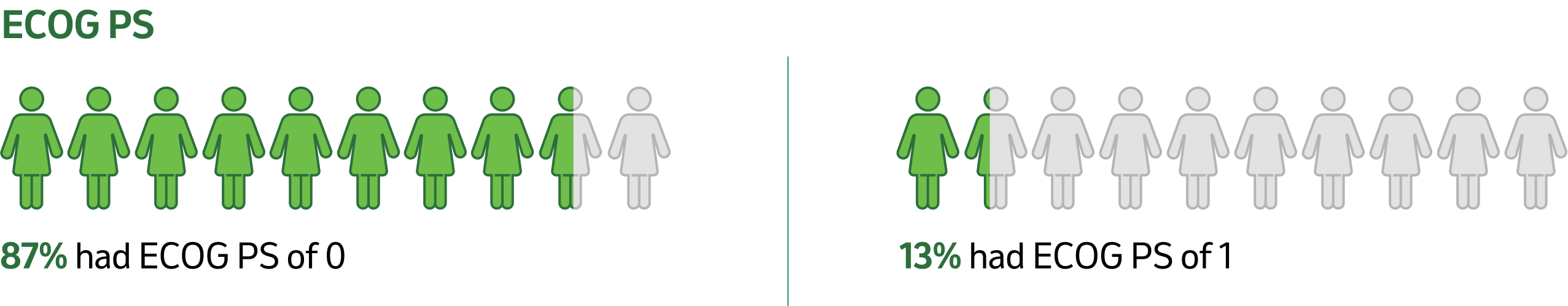

Tumour and nodes
7% were primary Tumour 1 (T1), 68% T2, 19% T3, and 7% T4.
49% were nodal involvement 0 (N0), 40% N1, 11% N2, and 0.2% N3.
83% were PD-L1 positive; 17% were PD-L1 negative.11
CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group Performance Status; N: nodal involvement; PD-L1: programmed death ligand 1.
The KEYNOTE-522 study outlines key patient characteristics that determine eligibility for the full neoadjuvant and adjuvant treatment regimen:5
Tumour size >1 cm but ≤2 cm in diameter with lymph node involvement
Tumour size >2 cm in diameter regardless of lymph node involvement
Regardless of tumour PD-L1 expression

For additional study eligibility criteria, see Schmid P et al., 2020.11
N−: lymph node negative; N+: lymph node positive; PD-L1: programmed death ligand 1.
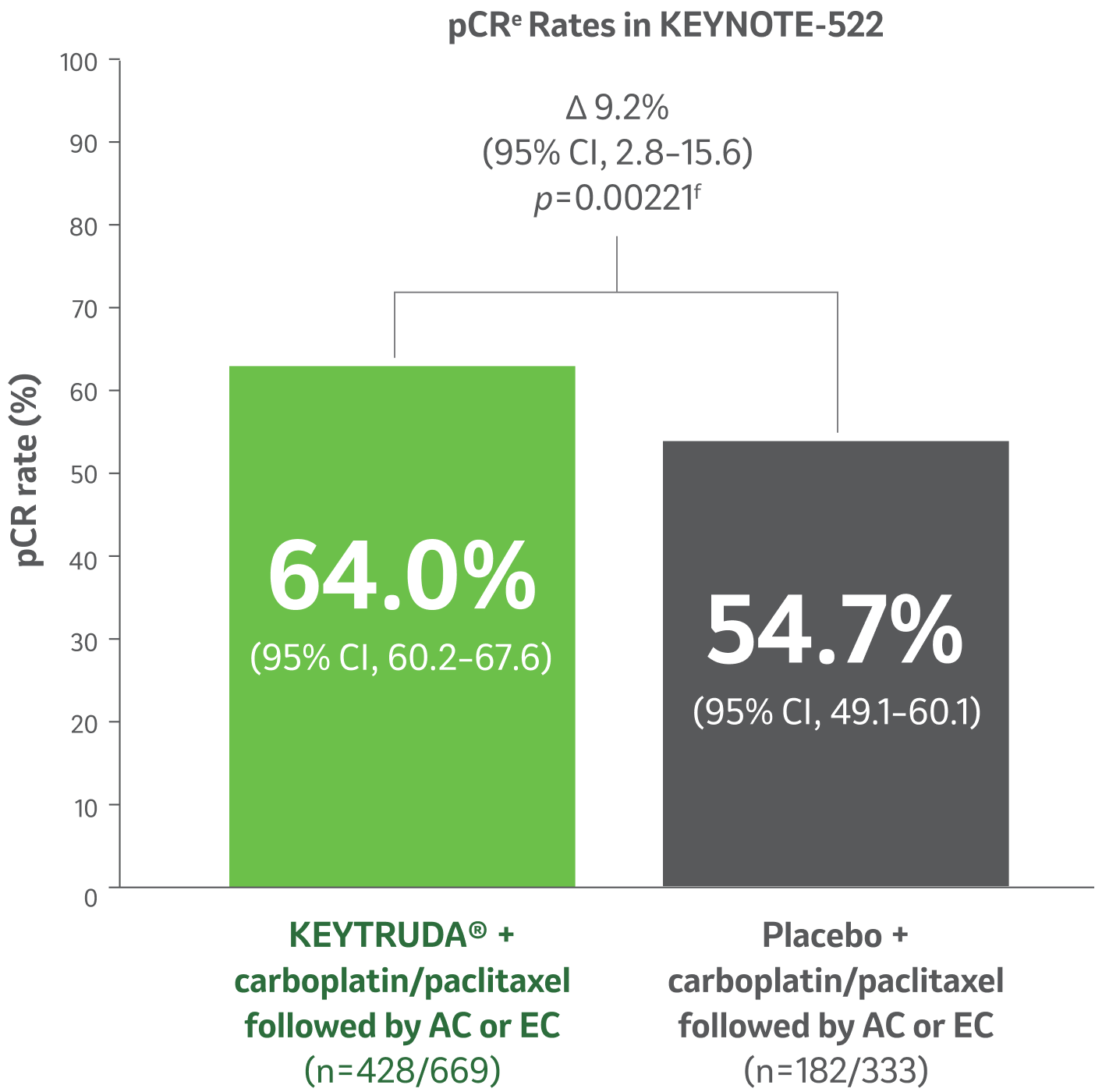
Pathological complete response (pCR) rate5
At the pre-specified final analysis (n=1002), 9.2% more patientsd achieved pCRe with KEYTRUDA® + chemotherapya regimen vs placebo + chemotherapya regimen (95% CI, 2.8–15.6; p=0.00221f).
Median follow-up 37.8 months (range: 2.7–48 months).

The KEYTRUDA® regimen demonstrated less invasive residual tissue in the breast or nodes at the time of definitive surgery and better patient outcomes compared to the placebo regimen.5
Event free survival (EFS):5
The study demonstrated a statistically significant improvement in EFS at its pre-specified interim analysis (median follow-up time for all patients of 37.8 months (range, 2.7–48.0 months), HR=0.63 (95% CI, 0.48–0.82; p-value 0.00031)).5
Updated EFS1
Median follow-up 75.1 months (range, 65.9–84.0 months).
LIMITATIONS: No formal statistical testing was planned for this protocol-specific analysis of EFS, and therefore, no conclusions can be drawn.
EFS at 75.1 Months in KEYNOTE-522
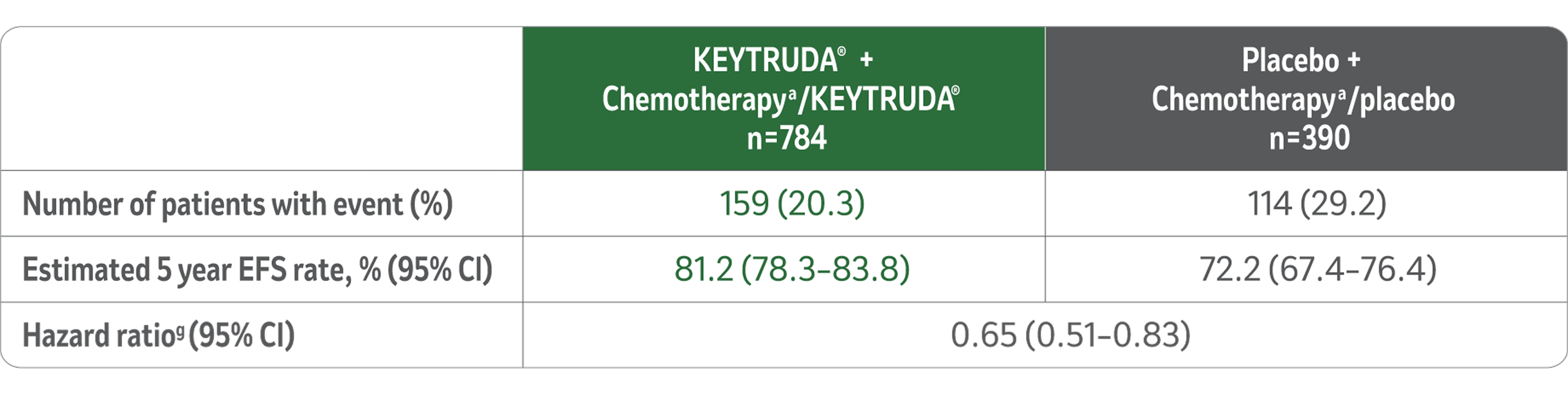
Kaplan-Meier Estimate of EFS of 75.1 Months
(Range, 65.9-84.0 Months)
Taken from Schmid et al. Overall survival with pembrolizumab in early-stage triple- negative breast cancer. N Engl J Med. 2024 Nov 28;391(21):1981-1991. Massachusetts Medical Society.
Reprinted with permission from Massachusetts Medical Society.

After a median follow up of ~ 6 years, for patients who received the complete KEYTRUDA® treatment regimen, event free survival (EFS) was consistent with results from previous analysis.
aChemotherapy: Carboplatin and paclitaxel followed by (doxorubicin or epirubicin) and cyclophosphamide.
dBased on Miettinen and Nurminen method stratified by nodal status, tumour size, and choice of carboplatin
eBased on a prespecified pCR final analysis (compared to a significance level of 0.0028).
fOne-sided p-value for testing. H0: difference in %=0 versus H1: difference in %>0.
gBased on Cox regression model with Efron’s method of tie handling with treatment as a covariate stratified by nodal status, tumour size, and choice of carboplatin.
AC: doxorubicin + cyclophosphamide; CI: confidence interval; EC: epirubicin + cyclophosphamide; EFS: event-free survival; HR: hazard ratio; pCR: pathological complete response.

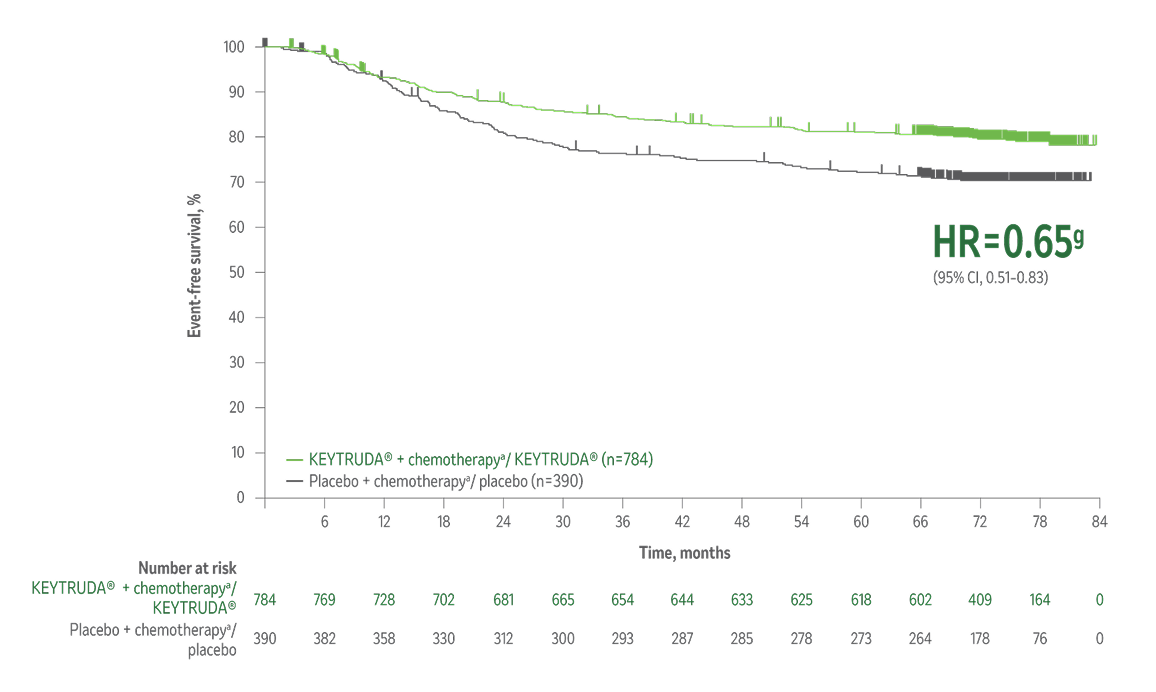
The results from the 7th interim analysis of the KEYNOTE-522 study show, for the first time, statistically significant improved overall survival in eTNBC patients with the KEYTRUDA® treatment regimen compared to the placebo regimen.1
Overall survival1
Superior overall survivalh in patients receiving KEYTRUDA® + chemotherapya/KEYTRUDA® vs placebo + chemotherapya/placebo.
34% reduction (HR=0.66i; 95% CI, 0.50–0.87; p=0.002j) in the risk of death with KEYTRUDA® + chemotherapya/KEYTRUDA® vs placebo + chemotherapya/placebo.
Median follow-up 75.1 months (range, 65.9–84.0 months).
Kaplan-Meier Curve for Overall Survivalj by Treatment Arm in KEYNOTE-522
(Intention-to-Treat Population)
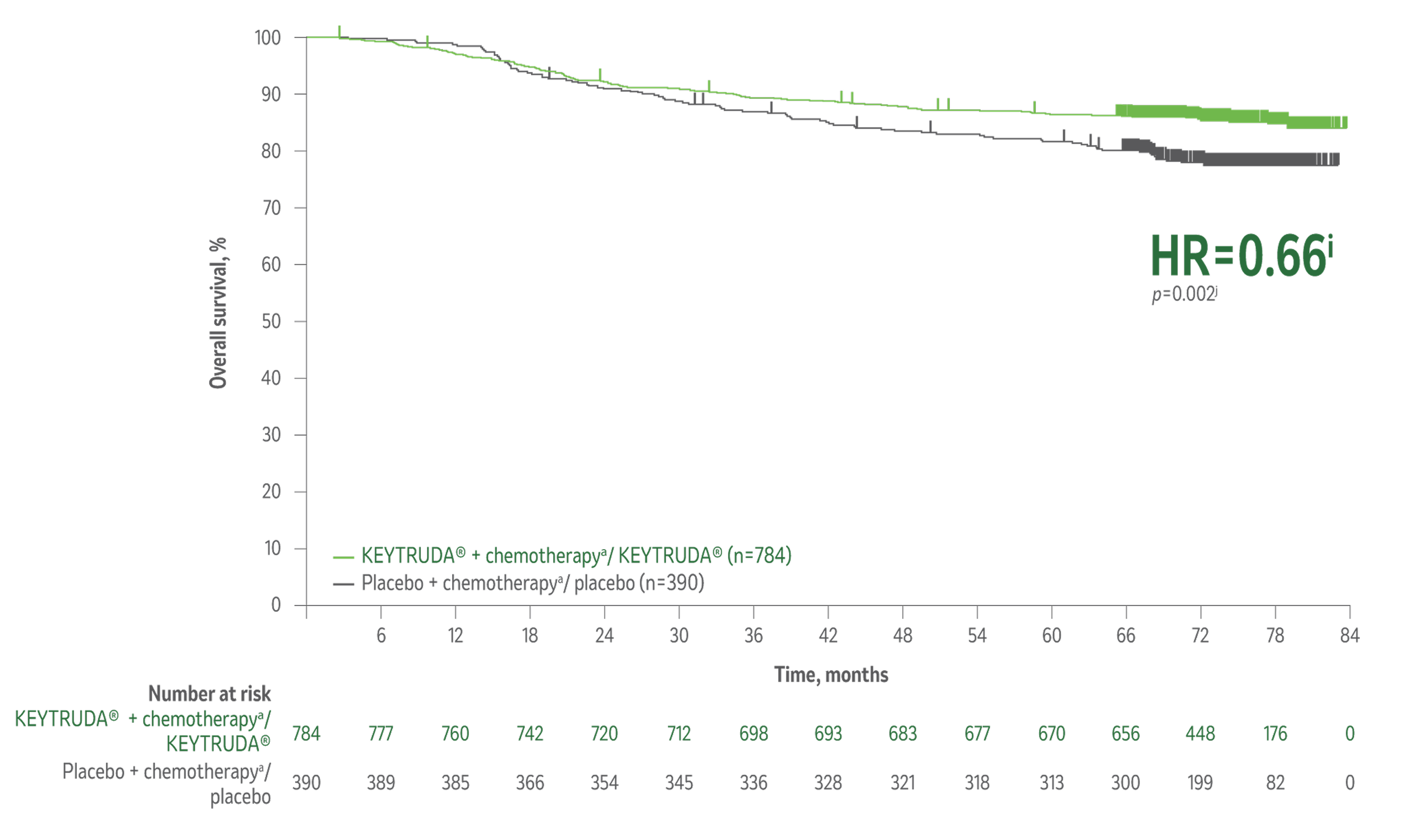
Taken from The New England Journal of Medicine, Schmid et al. Overall survival with pembrolizumab in early-stage triple- negative breast cancer. N Engl J Med. 2024;391(21):1981-1991. Copyright © 2024. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
The number of patients with an event (n/N) was 115/784 (14.7%) with KEYTRUDA® + chemotherapya/KEYTRUDA® vs 85/390 (21.8%) with placebo + chemotherapya/placebo.1

KEYTRUDA® is the first and only immunotherapy-based regimen to show a statistically significant improvement in OS as neoadjuvant treatment with the KEYTRUDA® combination regimen followed by KEYTRUDA® as a single agent after surgery compared to neoadjuvant chemotherapya followed by placebo after surgery in patients with high-risk eTNBC.1,5-10,12-14
Overall survival data in patients receiving KEYTRUDA® + chemotherapya/KEYTRUDA® vs placebo + chemotherapya/placebo across prespecified subgroups at median follow-up of 75.1 months.1
LIMITATIONS: KEYNOTE-522 was not powered to detect differences in the treatment effect in these subgroups, and no statistical testing was planned for this analysis; therefore, no conclusions can be drawn. Results from these exploratory subgroup analyses should be interpreted with caution because of the modest patient numbers, potential imbalances in baseline characteristics within the subgroups, and because the proportional hazard assumption did not hold in this analysis.
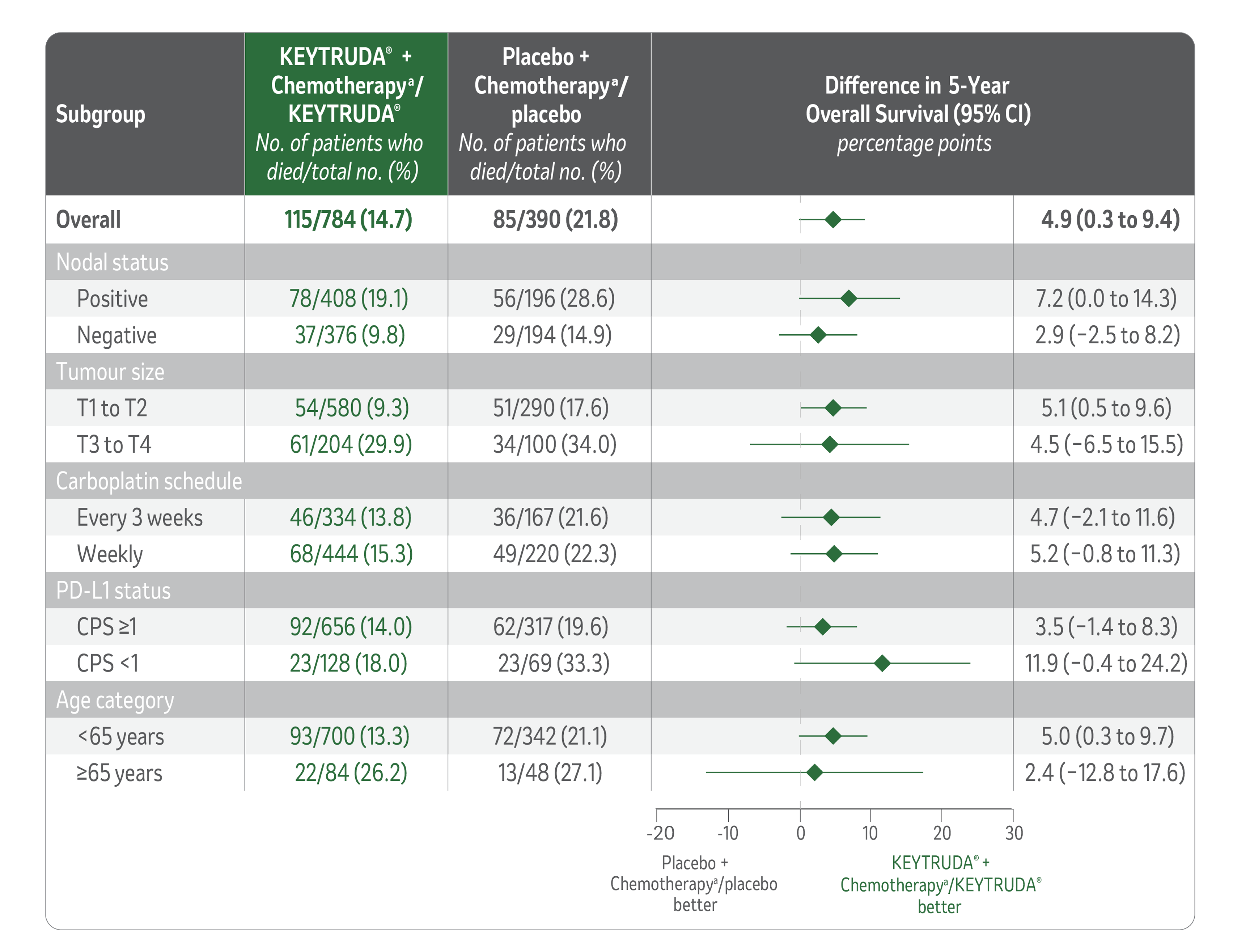
aChemotherapy: Carboplatin and paclitaxel followed by (doxorubicin or epirubicin) and cyclophosphamide.
hBased on a prespecified OS interim analysis (compared to a significance level of 0.00503)
iThe weighted average hazard ratio with weights of number of events before and after 2-year follow-up was 0.66.
jBased on log-rank test stratified by nodal status, tumour size, and choice of carboplatin.
CI: confidence interval; CPS: combined positive score; eTNBC: early triple-negative breast cancer; HR: hazard ratio; PD-L1: Programmed Death-Ligand 1.

KEYNOTE-522 demonstrated statistically significant improvement in overall survival, meaning more tomorrows for patients who received the complete KEYTRUDA® regimen compared to those who received the placebo regimen.1
Careful patient selection and monitoring for toxicities is essential.
The recommended dose of KEYTRUDA® in adults is either 200 mg Q3W or 400 mg Q6W5
When administering KEYTRUDA® as part of a combination with IV chemotherapy, KEYTRUDA® should be administered first as an IV infusion over 30 minutes. For the neoadjuvant and adjuvant treatment of high-risk early-stage TNBC, patients should be treated with neoadjuvant KEYTRUDA® in combination with chemotherapy for 8 doses of 200 mg Q3W or 4 doses of 400 mg Q6W or until disease progression that precludes definitive surgery or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA® as monotherapy for 9 doses of 200 mg Q3W or 5 doses of 400 mg Q6W or until disease recurrence or unacceptable toxicity. Patients who experience disease progression that precludes definitive surgery or unacceptable toxicity related to KEYTRUDA® as neoadjuvant treatment in combination with chemotherapy should not receive KEYTRUDA® monotherapy as adjuvant treatment.
No dose reductions of KEYTRUDA® are recommended. Withhold or discontinue KEYTRUDA® to manage moderate to severe adverse reactions per recommendations in the product labelling.
AUC: area under the curve; BSA: body surface area; IV: intravenous; Q1W: every 1 week; Q3W: every 3 weeks; Q6W: every 6 weeks; TNBC: triple-negative breast cancer.

Summary of safety profile of neoadjuvant KEYTRUDA® + chemotherapy followed by adjuvant KEYTRUDA® monotherapy for TNBC1
In patients with high-risk early-stage TNBC receiving KEYTRUDA® in combination with chemotherapya as a neoadjuvant treatment and continued as monotherapy adjuvant treatment, adverse events listed here occurred during or within 30 days after the treatment period (within 90 days for serious events) in descending order of frequency in the KEYTRUDA® + chemotherapya/KEYTRUDA® group. The as-treated population included all the patients who had undergone randomisation and received at least one trial drug, underwent surgery, or both. The severity of adverse events was graded according to the CTCAE, version 4.0, of the NCI.1
Adverse Events in the Combined Neoadjuvant and Adjuvant Phases
(Median Follow-Up 75.1 Months)1
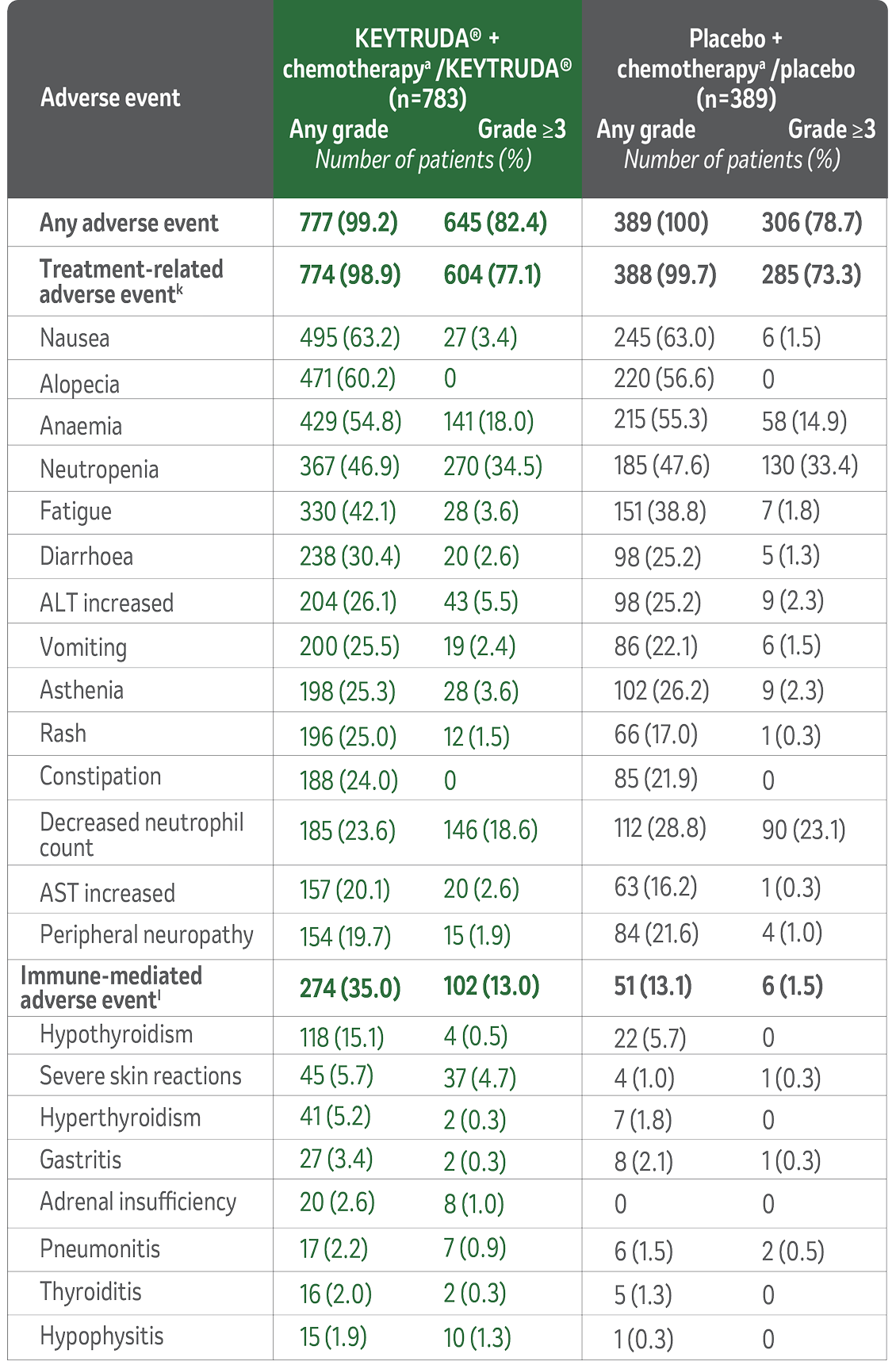
Grade 5 treatment-related adverse events were sepsis and multiple organ dysfunction syndrome (n=1); pneumonitis (n=1); pulmonary embolism (n=1); autoimmune encephalitis (n=1) in the KEYTRUDA® + chemotherapya/KEYTRUDA® group; and septic shock (n=1) in the placebo + chemotherapya/placebo group.
Grade 5 immune-mediated adverse events were pulmonary embolism (n=1) and autoimmune encephalitis (n=1) in the KEYTRUDA® + chemotherapya/KEYTRUDA® group.
aChemotherapy: Carboplatin and paclitaxel followed by (doxorubicin or epirubicin) and cyclophosphamide.
kTreatment-related adverse events were events that were attributed to a trial treatment by the investigators. Treatment-related adverse events that occurred in at least 20% of the patients in either treatment group are reported. Patients may have had more than one event.
lImmune-mediated adverse events were determined according to a list of terms specified by the sponsor, regardless of attribution to any trial treatment by the investigators. Immune-mediated adverse events that occurred in at least 15 patients in either treatment group are reported.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CTCAE: Common Terminology Criteria for Adverse Events; NCI: National Cancer Institute; TNBC: triple-negative breast cancer.
You can increase your patients’ chances for better outcomes by partnering with an MDT and offering them more support through each stage of their treatment regimen.15-18
Most early breast cancer cases can be cured by multimodality treatment, although cure rates vary by clinical stage and subtype.15

The decision to use neoadjuvant and/or adjuvant therapy should be made by an MDT.16

MDT decision-making increases the chances for better outcomes for your patients.17,18

The ESMO guidelines recommend an MDT approach.15
Neoadjuvant KEYTRUDA® + chemotherapya followed by adjuvant KEYTRUDA® monotherapy is recommended in:
Patients with cT2c-T4 node negative diseasem
Patients with any node positive (stage II–III) TNBCm

Adjuvant KEYTRUDA® monotherapy is recommended for all patients who have received KEYTRUDA® + chemotherapya in the neoadjuvant setting, regardless of pCR status.15
ESMO guidance on KEYTRUDA® use in eTNBC patients can be found on the ESMO website.
aChemotherapy: Carboplatin and paclitaxel followed by (doxorubicin or epirubicin) and cyclophosphamide.
mUnless there are risk factors for excessive ICI-associated immune toxicity.
KEYTRUDA® (Pembrolizumab) Selected Safety Information (SSI)
INDICATION:
Melanoma: KEYTRUDA as monotherapy is indicated for the treatment of adults and adolescents aged 12 years and older with advanced (unresectable or metastatic) melanoma. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults and adolescents aged 12 years and older with Stage IIB, IIC or III melanoma and who have undergone complete resection.
Non-small cell lung carcinoma (NSCLC): KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations. KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous non-small cell lung carcinoma in adults whose tumours have no EGFR or ALK positive mutations. KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous non-small cell lung carcinoma in adults. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic non-small cell lung carcinoma in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA. KEYTRUDA is indicated for the treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC in combination with platinum containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery. KEYTRUDA, as monotherapy is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with Stage IB (T2a ≥4 cm), II, or IIIA NSCLC.
Classical Hodgkin lymphoma (cHL): KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option.
Urothelial carcinoma: KEYTRUDA, in combination with enfortumab vedotin, is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy. KEYTRUDA is indicated for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. This indication was approved based on complete response rate and duration of response. Continued approval of this indication depends on verification and description of benefit in confirmatory trials.
Head and neck squamous cell carcinoma (HNSCC): KEYTRUDA, as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a CPS ≥1. KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a ≥50% TPS and progressing on or after platinum-containing chemotherapy.
Renal cell carcinoma (RCC): KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of advanced renal cell carcinoma in adults. KEYTRUDA, in combination with lenvatinib, is indicated for the first-line treatment of advanced renal cell carcinoma in adults. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
Microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) cancers
Colorectal cancer (CRC): KEYTRUDA as monotherapy is indicated for adults with MSI-H or dMMR colorectal cancer in the following settings:
- First-line treatment of metastatic colorectal cancer.
- Treatment of unresectable or metastatic colorectal cancer after previous fluoropyrimidine-based combination therapy.
Non-colorectal cancers: KEYTRUDA as monotherapy is indicated for the treatment of the following MSI-H or dMMR tumours in adults with:
- Advanced or recurrent endometrial carcinoma, who have disease progression on or following prior treatment with a platinum-containing therapy in any setting and who are not candidates for curative surgery or radiation.
- Unresectable or metastatic gastric, small intestine, or biliary cancer, who have disease progression on or following at least one prior therapy.
Gastric or gastro-oesophageal junction (GEJ) adenocarcinoma: KEYTRUDA, in combination with trastuzumab, fluoropyrimidine and platinum-containing chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric or gastro-oesophageal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥1 as determined by a validated test.
KEYTRUDA, in combination with fluoropyrimidine and platinum-containing chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or gastro-oesophageal junction (GEJ) adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥1.
Oesophageal carcinoma: KEYTRUDA, in combination with platinum and fluoropyrimidine-based chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesophageal junction adenocarcinoma, in adults whose tumours express PD-L1 with a CPS ≥10.
Triple-negative breast cancer (TNBC): KEYTRUDA, in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advanced, or early-stage triple-negative breast cancer at high risk of recurrence. KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple-negative breast cancer in adults whose tumours express PD-L1 with a CPS ≥10 and who have not received prior chemotherapy for metastatic disease.
Endometrial carcinoma (EC): KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of adult patients with advanced endometrial carcinoma that is mismatch repair proficient (pMMR) or not MSI-H, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation.
KEYTRUDA, in combination with carboplatin and paclitaxel, followed by KEYTRUDA as monotherapy, is indicated for the treatment of patients with primary advanced or recurrent endometrial carcinoma.
Cervical cancer: KEYTRUDA, in combination with chemoradiotherapy (external beam radiation therapy followed by brachytherapy), is indicated for the treatment of FIGO 2014 Stage III-IVA locally advanced cervical cancer in adults who have not received prior definitive therapy.
KEYTRUDA, in combination with chemotherapy with or without bevacizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tumours express PD-L1 with a CPS ≥1.
Biliary Tract Carcinoma: KEYTRUDA, in combination with gemcitabine and cisplatin, is indicated for the treatment of patients with locally advanced unresectable or metastatic biliary tract carcinoma (BTC).
DOSAGE & ADMINISTRATION: KEYTRUDA is administered 30 minutes intravenous infusion. No dose reductions of KEYTRUDA are recommended. KEYTRUDA should be withheld or discontinued to manage adverse reactions.
POSOLOGY:
The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes.
The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL or patients aged 12 years and older with melanoma is 2 mg/kg bodyweight (bw) (up to a maximum of 200 mg), every 3 weeks administered as an intravenous infusion over 30 minutes.
For use in combination, see the Summary of Product Characteristics (SmPC) for the concomitant therapies.
For urothelial carcinoma patients treated with KEYTRUDA in combination with enfortumab vedotin, administer KEYTRUDA after enfortumab vedotin when given on the same day.
For urothelial carcinoma patients treated with KEYTRUDA in combination with enfortumab vedotin, the recommended initial dose of enfortumab vedotin is 1.25 mg/kg (up to a maximum of 125 mg for patients ≥100 kg) as an intravenous solution on Days 1 and 8 of a 21-day cycle until disease progression or unacceptable toxicity.
Patients should be treated with KEYTRUDA until disease progression or unacceptable toxicity (and up to maximum duration of therapy if specified for an indication). Atypical responses (i.e. an initial transient increase in tumour size or small new lesions within the first few months followed by tumour shrinkage) have been observed. It is recommended to continue treatment for clinically stable patients with initial evidence of disease progression until disease progression is confirmed.
For the adjuvant treatment of melanoma, NSCLC, or RCC, KEYTRUDA should be administered until disease recurrence, unacceptable toxicity, or for a duration of up to one year.
For the neoadjuvant and adjuvant treatment of resectable NSCLC, patients should be treated with neoadjuvant KEYTRUDA in combination with chemotherapy for 12 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA as monotherapy for 39 weeks or until disease recurrence or unacceptable toxicity.
For the neoadjuvant and adjuvant treatment of TNBC, patients should be treated with neoadjuvant KEYTRUDA in combination with chemotherapy for 8 doses of 200 mg every 3 weeks or 4 doses of 400 mg every 6 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA as monotherapy for 9 doses of 200 mg every 3 weeks or 5 doses of 400 mg every 6 weeks or until disease recurrence or unacceptable toxicity. Patients who experience disease progression that precludes definitive surgery or unacceptable toxicity related to KEYTRUDA as neoadjuvant treatment in combination with chemotherapy should not receive KEYTRUDA monotherapy as adjuvant treatment.
CONTRAINDICATIONS: Hypersensitivity to the active substance or to any of the excipients.
PRECAUTIONS/WARNINGS: KEYTRUDA can cause Immune-mediated adverse reactions, including severe and fatal cases. Immune-mediated Pneumonitis, Immune-mediated Colitis, Immune-mediated Hepatitis, Immune-mediated Nephritis, Immune-mediated Endocrinopathies (as adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, diabetic ketoacidosis, hypothyroidism, and hyperthyroidism) & Immune-mediated Skin adverse reactions. KEYTRUDA can cause other immune-mediated adverse reactions as: uveitis, arthritis, myositis, myocarditis, pancreatitis, Guillain-Barré syndrome, myasthenic syndrome, haemolytic anaemia, sarcoidosis, encephalitis, myelitis, vasculitis, cholangitis sclerosing, gastritis, cystitis noninfective and hypoparathyroidism. KEYTRUDA can cause severe infusion-related reactions including hypersensitivity and anaphylaxis. Solid organ transplant rejection has been reported in the post-marketing setting in patients treated with PD-1 inhibitors. Cases of graft-versus-host-disease (GVHD) and hepatic veno-occlusive disease (VOD) have been observed in patients with cHL undergoing allogeneic HSCT after previous exposure to KEYTRUDA. In patients with a history of allogeneic HSCT, acute GVHD, including fatal GVHD, has been reported after treatment with KEYTRUDA. KEYTRUDA should not be used during pregnancy unless the clinical condition of the woman requires treatment with KEYTRUDA.
MOST COMMON ADVERSE EVENTS:
Monotherapy: anemia, hypothyroidism, decreased appetite, headache, dyspnea, cough, diarrhea, abdominal pain, nausea, vomiting,constipation, pruritus, rash, musculoskeletal pain, arthralgia, fatigue, asthenia, oedema, pyrexia.
In combination with chemotherapy: neutropenia, anemia, thrombocytopenia, hypothyroidism, hypokalemia, decreased appetite, insomnia, neuropathy peripheral, headache, dyspnea, cough, nausea, diarrhea, vomiting, abdominal pain, constipation, alopecia, rash, pruritus, arthralgia, musculoskeletal pain, fatigue, asthenia, pyrexia, alanine aminotransferase increased, aspartate aminotransferase increased.
In combination with Axitinib or Lenvatinib: urinary tract infection, anemia, hypothyroidism, decreased appetite, headache, dysgeusia, hypertension, dyspnea, cough, diarrhea, abdominal pain, nausea, vomiting, constipation, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pain in extremity, fatigue, asthenia, oedema, pyrexia, lipase increased, alanine aminotransferase increased, aspartate aminotransferase increased, blood creatinine increased.
Please refer to the full Summary of Product Characteristics (SmPC) of KEYTRUDA® for more information
EFS: event-free survival; ESMO: European Society for Medical Oncology; eTNBC: early triple-negative breast cancer; MDT: multidisciplinary team; pCR: pathological complete response.
References:
- Schmid P, Cortes J, Dent R, et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N Engl J Med. 2024;391(21):1981-1991.
- Stuart-Harris R, Dahlstrom JE, Gupta R, et al. Recurrence in early breast cancer: analysis of data from 3,765 Australian women treated between 1997 and 2015. Breast. 2019;44:153-9.
- Gupta GK, Collier AL, Lee D, et al. Perspectives on triple-negative breast cancer: current treatment strategies, unmet needs, and potential targets for future therapies. Cancers (Basel). 2020;12(9).
- Newton EE, Mueller LE, Treadwell SM, et al. Molecular targets of triple-negative breast cancer: where do we stand? Cancers (Basel). 2022;14(3).
- KEYTRUDA®. Summary of product characteristics.
- Opdivo®. Summary of product characteristics.
- Libtayo®. Summary of product characteristics.
- Tevimbra®. Summary of product characteristics.
- ZYNYZ®. Summary of product characteristics.
- Jemperli®. Summary of product characteristics.
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-21.
- Tecentriq®. Summary of product characteristics.
- Imfinzi®. Summary of product characteristics.
- Bavencio®. Summary of product characteristics.
- Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35(2):159-82.
- Cain H, Macpherson IR, Beresford M, et al. Neoadjuvant therapy in early breast cancer: treatment considerations and common debates in practice. Clin Oncol (R Coll Radiol). 2017;29(10):642-52.
- Croke JM, El-Sayed S. Multidisciplinary management of cancer patients: chasing a shadow or real value? An overview of the literature. Curr Oncol. 2012;19(4):e232-8.
- Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
SA-OBR-00092 | 05/8/2027