KN 564

Study Design
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial1
The randomized, double-blind, placebo-controlled, phase 3 KEYNOTE-564 study was designed to investigate adjuvant pembrolizumab monotherapy versus placebo after nephrectomy for participants with localized renal cell carcinoma or after nephrectomy and metastasectomy for participants with M1 stage renal cell carcinoma.
Inclusion Criteria1
- Adults aged 18 years or older.
- Had undergone surgery for histologically confirmed renal cell carcinoma with a clear cell component with or without sarcomatoid features.
- Participants were categorized as having an intermediate-high risk of disease recurrence (pathological tumour stage 2 [pT2] with nuclear grade 4 or sarcomatoid differentiation, no nodal involvement [N0], and no metastasis [M0]; or pT3, any grade, N0, M0), high risk of disease recurrence (pT4, any grade, N0, M0; or any pT, any grade, N+, M0), or M1 stage with no evidence of disease after complete resection of oligometastases synchronously or within 1 year of nephrectomy (M1 with no evidence of disease).
- Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1.
- Had undergone surgery within the 12 weeks before randomization.
- Had disease-free status at baseline according to investigator assessment.
- Had received no previous systemic therapy for advanced renal cell carcinoma; and provided adequate tissue samples for PD-L1 assessment from the nephrectomy (if they received nephrectomy only), from both the nephrectomy and metastasectomy (if done synchronously), or from the metastasectomy (if done after nephrectomy; nephrectomy tissue sample had to also be provided if available).
Exclusion Criteria1
- If they had any major surgery other than nephrectomy or metastasectomy within the 12 weeks before randomisation.
- Had received previous radiotherapy for renal cell carcinoma.
- Had received previous anti-PD-1, anti-PD-L1, anti-PD-L2, or other co-inhibitory T-cell receptor therapy.
- Had pre-existing brain or bone metastases.
- Participants with residual disease after nephrectomy.
Stratification Factors1
- Metastatic status (M0 vs M1 NED).
- Further stratification within No metastasis subgroup (M0) by ECOG performance status (scores range 0-5, with higher score indicating greater disability).
- Geographic location (US vs non-US).
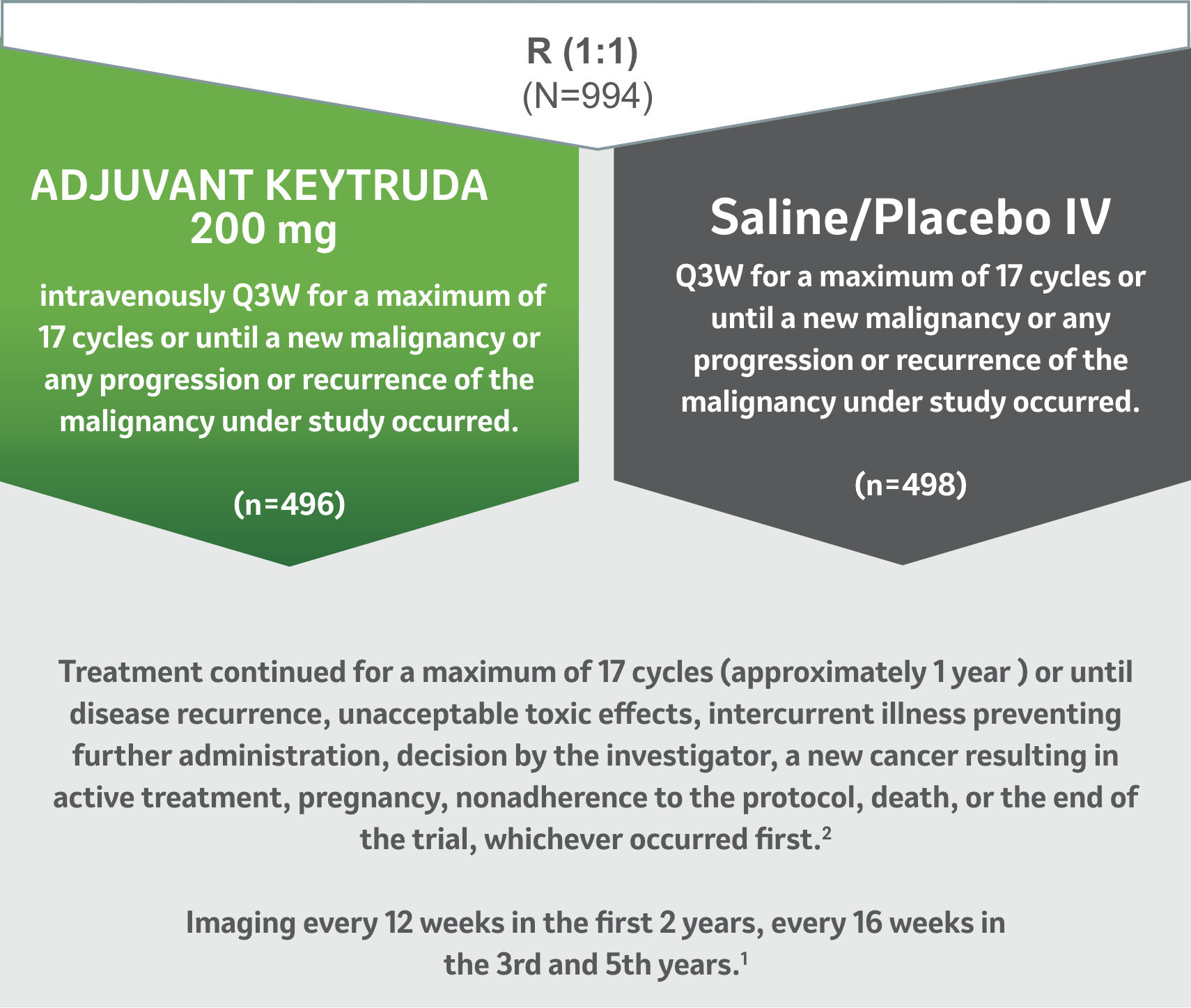
- Primary efficacy outcome measure: Investigator-assessed disease-free survival (DFS).1
- Key secondary outcome measure: overall survival (OS).1
- Median follow-up time: 30.1 months (range: 20.8–47.5 months).1
Baseline Characteristics
Intermediate-High Risk:2

pT2 with Grade 4
or sarcomatoid features
pT3 with any grade without
nodal involvement (N0) or
distant metastates (M0)
High Risk:2

pT4, with any
grade N0 and M0

Any pT stage, any grade
with nodal involvement
(N+) and M0
M1 NED:2
Patients with metastatic disease who had undergone complete resection of primary and metastatic lesions
Exclusion Criteria:1
- If they had any major surgery other than nephrectomy or metastasectomy within the 12 weeks before randomisation.
- Had received previous radiotherapy for renal cell carcinoma.
- Had received previous anti-PD-1, anti-PD-L1, anti-PD-L2, or other co-inhibitory T-cell receptor therapy.
- Had pre-existing brain or bone metastases.
- Participants with residual disease after nephrectomy.
ECOG PS = Eastern Cooperative Oncology Group performance status; M0 = no metastasis; M1 = with distant metastases; NED = no evidence of disease; Q3W = every 3 weeks; RCC = renal cell carcinoma.
References:
- Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, doubleblind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133-1144. doi:10.1016/S1470-2045(22)00487- 9
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–69.
Disease-Free Survival
KEYTRUDA Significantly Reduced the Risk of Disease Recurrence, or Death by 32% Compared with Saline/Placebo IV1
Median time from randomization to data cutoff: 24.1 months (range: 14.9 – 41.5 months)1

In Patients with RCC at Increased
Risk of Recurrence Following
Nephrectomy, or Following Nephrectomy
and Resection of Metastatic Lesions.

Risk of disease recurrence, or death significantly reduced
with KEYTRUDA compared
with saline/placebo IV
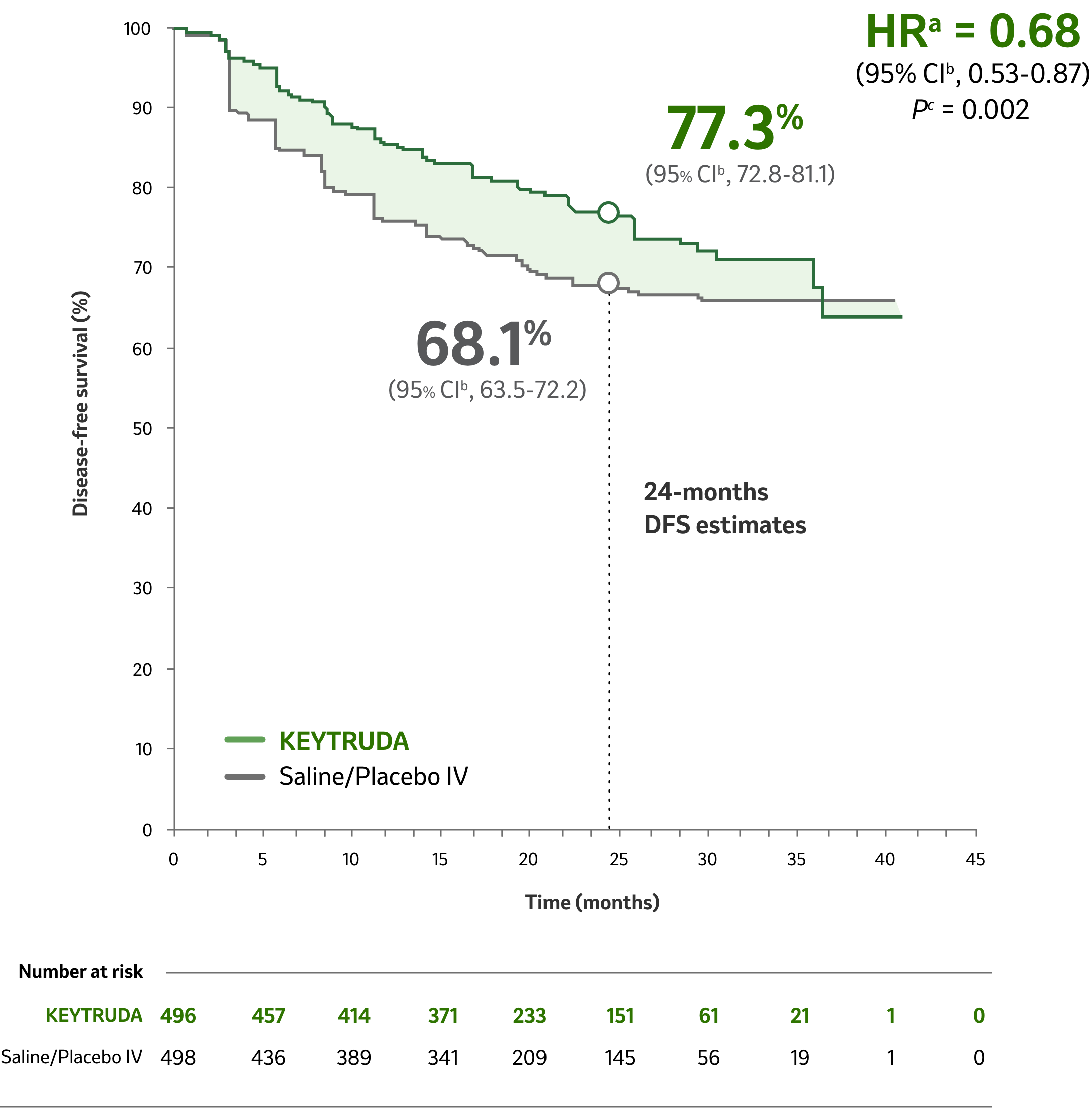
aBased on the stratified Cox proportional hazard model.1
bBased on stratified log-rank test.1
cThe P-value boundary for significance was 0.0114 (one-sided ) for the analysis of disease-free survival.1
- Events observed: 109/496 (22%) with KEYTRUDA vs 151/498 (30%) with saline/placebo IV.
- Median DFS: Not reached for KEYTRUDA or saline/placebo IV.
- At the time of the DFS analysis, OS data were not mature.
Follow-up Analysis: Median 30.1 Months2
Limitations: No formal statistical testing was performed for the updated analysis, and, therefore, no conclusions can be drawn.2
Median time from randomization to data cutoff: 30.1 months (range: 25.7−36.7 months)2

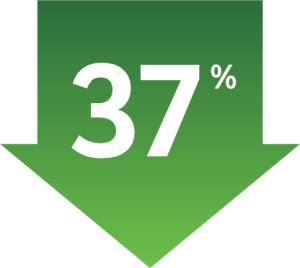
Reduction in the risk of
disease recurrence, or death
compared with saline/placebo IV
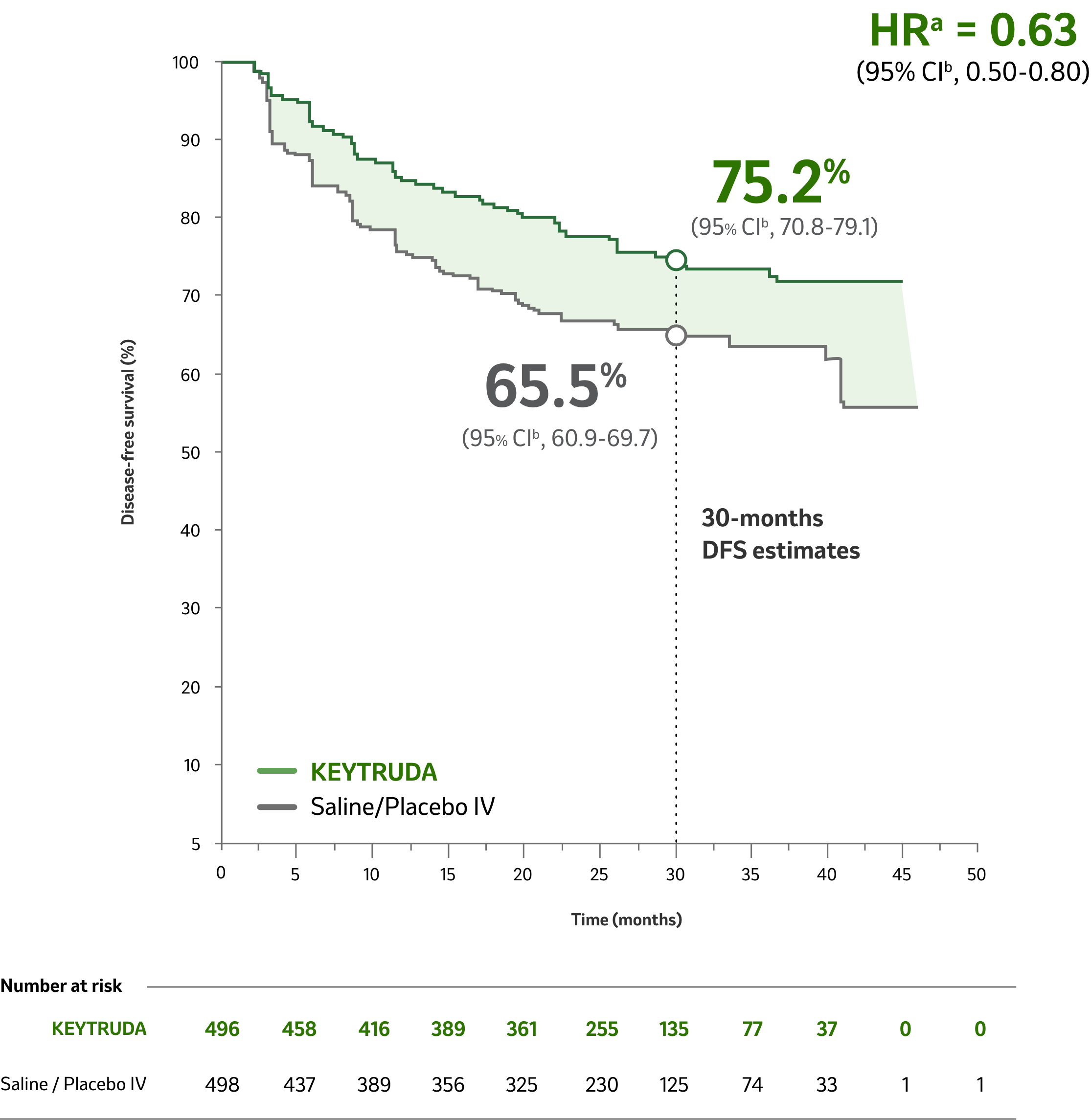
a Based on the stratified Cox proportional hazard model.1
b Based on stratified log-rank test.1
HR = hazard ratio; CI = confidence interval.
References:
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694.
- Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133-1144. doi:10.1016/S1470-2045(22)00487-9
Safety Profile
Any-Cause Adverse Events with an Incidence of at Least 10% in Either Group
(As-Treated Population).1
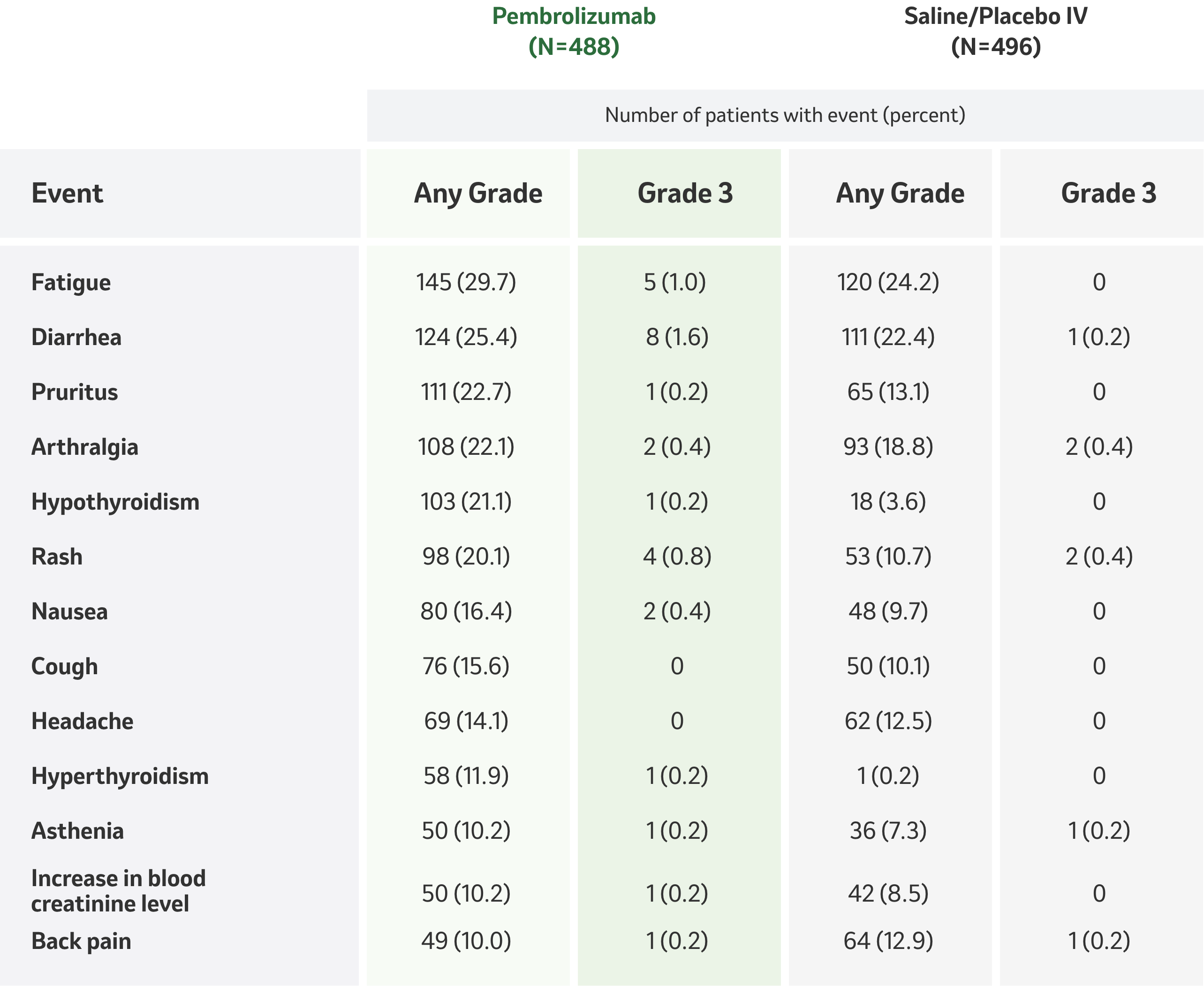
*No adverse events of grade 4 or 5 occurred in the least 10% of the patients in either group.
Adapted from Table 3, Choueiri TK, Tomczak P, Park SH, et al. N Engl J Med. 2021;385(8):683–69
Adverse Reactions1
- In the as-treated population, 20.7% of the patients in the pembrolizumab group and 2.0% of those in the saline/placebo IV group discontinued the respective trial regimen because of adverse events, the majority of which were nonserious.
- Among the patients who received pembrolizumab, only an increase in the alanine aminotransferase level (in 1.6%), adrenal insufficiency (in 1.0%), and colitis (in 1.0%) led to treatment discontinuation in 1.0% or more of the patients. Dose interruptions due to an adverse event were reported in 25.8% of the patients in the pembrolizumab group and in 14.9% of those in the saline/placebo IV group.
- At least one treatment-related serious adverse event occurred in 12.1% of the patients who received pembrolizumab and in 0.2% of those who received saline/placebo IV.
- The most common adverse events of any cause in the two groups were fatigue (in 29.7% of the patients who received pembrolizumab and in 24.2% of those who received saline/placebo IV), diarrhea (in 25.4% and 22.4%, respectively), pruritus (in 22.7% and 13.1%), and arthralgia (in 22.1% and 18.8%).
- The median duration of the trial regimen was 11.1 months (range, 0.0 to 14.3) in the pembrolizumab group and 11.1 months (range, 0.0 to 15.4) in the saline/placebo IV group.
- The as-treated population included all the patients who received at least one dose of pembrolizumab or saline/ placebo IV. Adverse events were recorded from randomization through 30 days after the discontinuation pembrolizumab or saline/placebo IV. Serious adverse events were defined as any adverse event that resulted in death, was life-threatening, resulted in inpatient hospitalization, resulted in persistent or significant disability or incapacity, was a congenital anomaly or birth defect, or was judged by the investigator to be a serious adverse event. Serious adverse events were recorded from randomization through 90 days after the discontinuation of pembrolizumab or saline/placebo IV.
Patient Eligibility Criteria
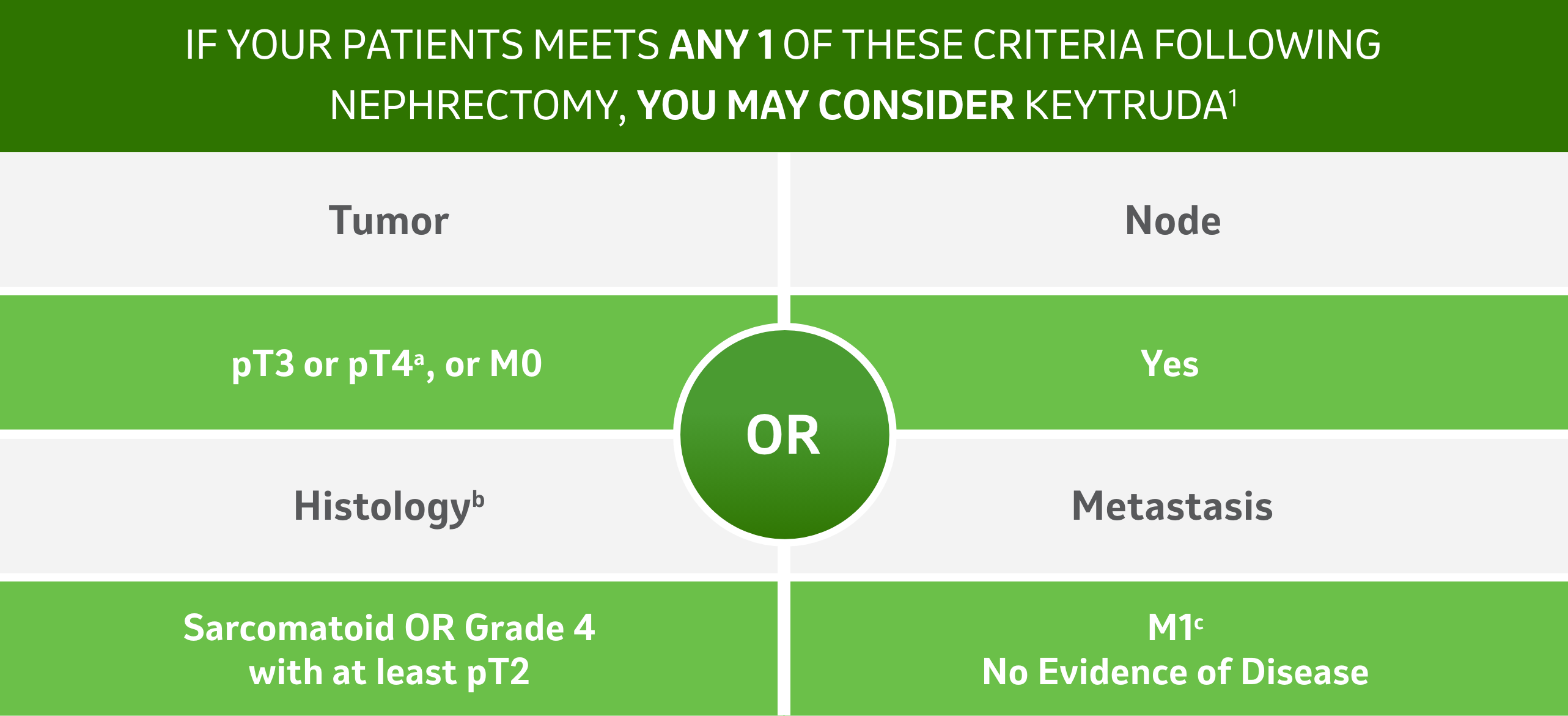
Adapted from Choueiri TK, et al.
a Complete resection of disease
b All patients in KN-564 Trial were presented with Clear Cell Histology
c Metastasectomy with complete resection of disease
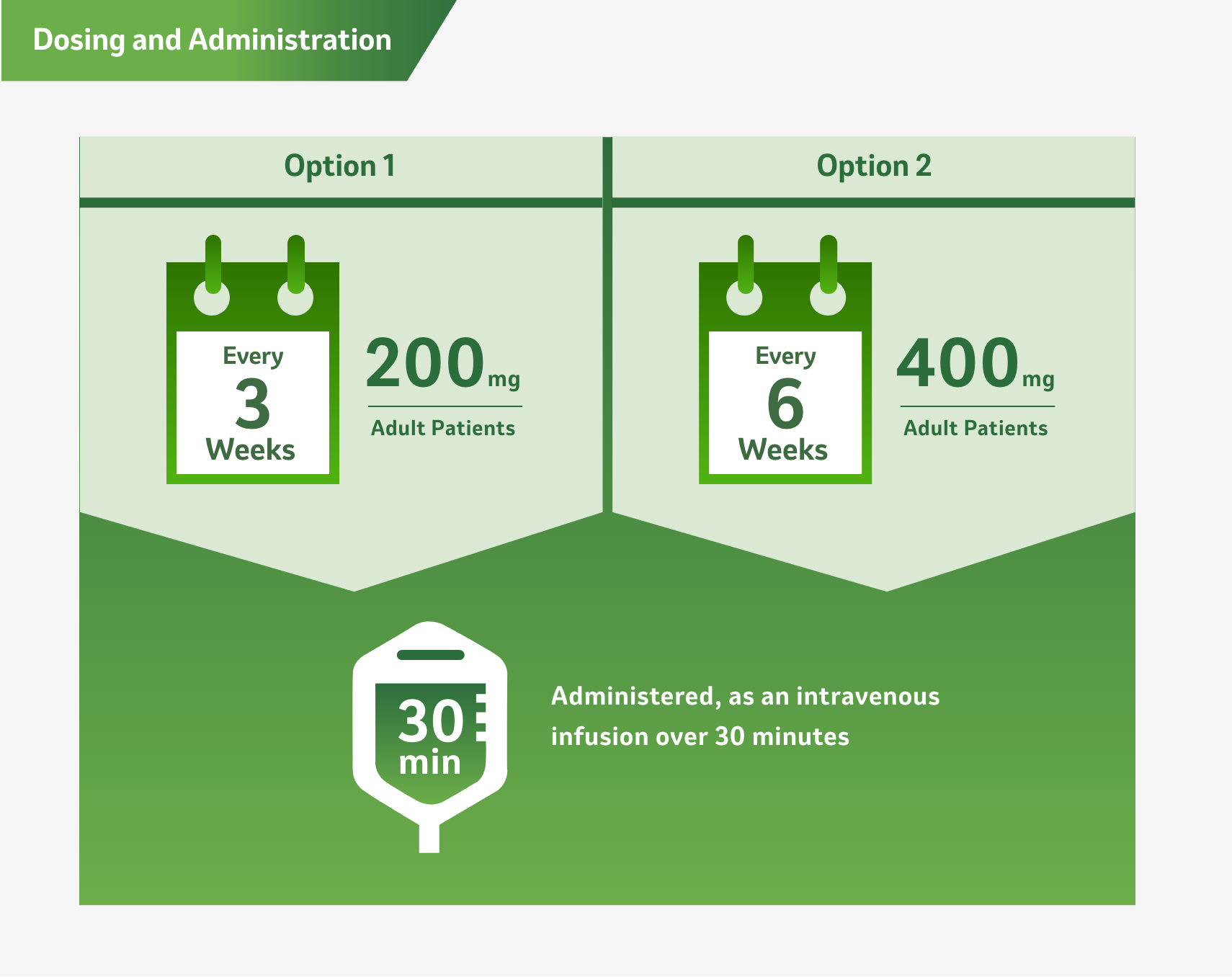
Dosing and Administration2
Patients received 200 mg of KEYTRUDA by intravenous infusion every 3 weeks or saline/placebo IV for up to 1 year or until disease recurrence or unacceptable toxicity.
NCI CTCAE = National Cancer Institute Common Terminology Criteria for Adverse Events; ALT = alanine aminotransferase.
References:
- Choueiri TK, Tomczak P, Park SH, et al; for the KEYNOTE-564 investigators. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694.
- KEYTRUDA® USPI
AE-JRC-00002 | Expiry Date: 17-11-2025