KSA-Recommending HPV Vaccine
HCP (health care provider) recommendation is a key determinant of HPV vaccine uptake1

High-quality recommendations by physicians can increase vaccination series initiation by 3-fold and completion by 9-fold.2
Your recommendation is significantly related to both intention to receive the vaccine and to receiving a full dose of the vaccine among those who had not been vaccinated (p<0.001).3
There is a large knowledge gap on eligible vaccination age groups3
Just as it is beneficial to discuss vaccination with the parents of children who are in the recommended age group, there is a need to encourage provider conversation with adults who can benefit from vaccination.3

An online survey conducted among 421 US adults 18-45 years old in April 2019 found 96 % of participants were unaware that the HPV vaccine had been approved for adults.3
Only 4 % of those surveyed were aware of the 2018 FDA (Food and Drug Administration) changes about approval for adults 26–45 years.3
Survey results3
30 %
Received provider recommendation for the HPV vaccine

35 %
Provider started a conversation about the HPV vaccine in general

26.4 %
Received at least one dose of the HPV vaccine

19.5 %
Received the full three doses

A survey study was conducted to examine knowledge about changes to the FDA’s approval of the HPV vaccine for adults ages 26-45 years old and to explore which IBM constructs are significantly related to HPV vaccine intention. A sample was recruited through a national paneling company. 420 participants were included in the study. The inclusion criteria were being between 18-45 years old and currently living in the United States. Eligible participants completed a 15-minute study administered through software. Attitudes, perceived norms, and perceived control were measured on 5-point Likert scales (1=strongly disagree, 5=strongly agree), and knowledge questions were developed to examine a participant’s knowledge of the number of doses required per age group and currently approved age groups by the FDA. Averages across items were calculated to create composite scores for each construct.3
Changing patient perspectives on HPV vaccines

“I trust my doctor and his group to tell me what I should know.”4
Male participants in a 2023 qualitative study noted that they considered their HCPs to be trusted sources of HPV vaccine information.4
Despite being preferred sources of HPV vaccine information among the men in this study, most participants had never discussed HPV vaccination with an HCP.4
Two main reasons why HPV vaccination was not discussed with a healthcare provider4:

HCP recommendations tend to be less frequent and of lower quality when the patient is male

Many U.S. physicians are unaware of the new shared clinical decision-making guidance for adults aged 27-45

Nurse practitioners also play a role in HPV vaccine recommendations!5
Nurses are at an advantage in the clinical setting to narrow the gap between education and vaccine intention.5
Key points to include in conversation with patients about HPV vaccination6
Explain when to vaccinate and why the HPV vaccine is important

Proactively explain potential side-effects of the HPV vaccine

Promote safety of the HPV
vaccine

Focus on cancer prevention
Emphasize long
lasting protection
Keep in mind that the long-term goal for these conversations is vaccine acceptance, and it may take more than one visit to win your patient’s confidence.6
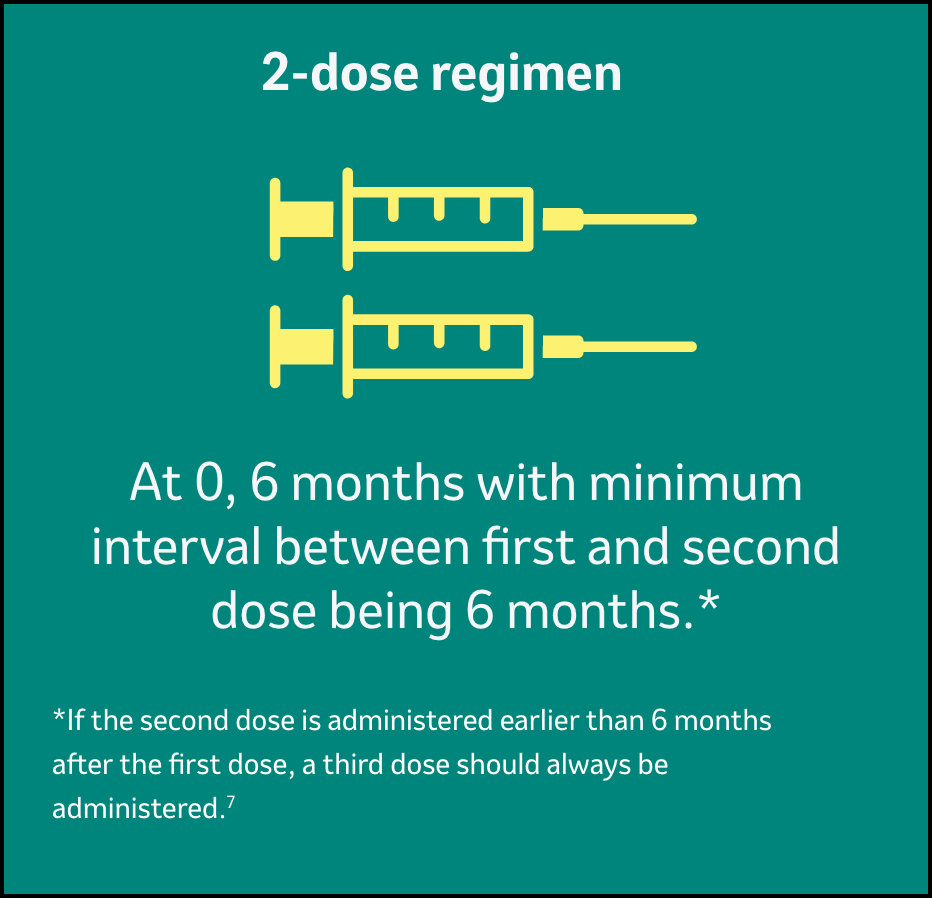
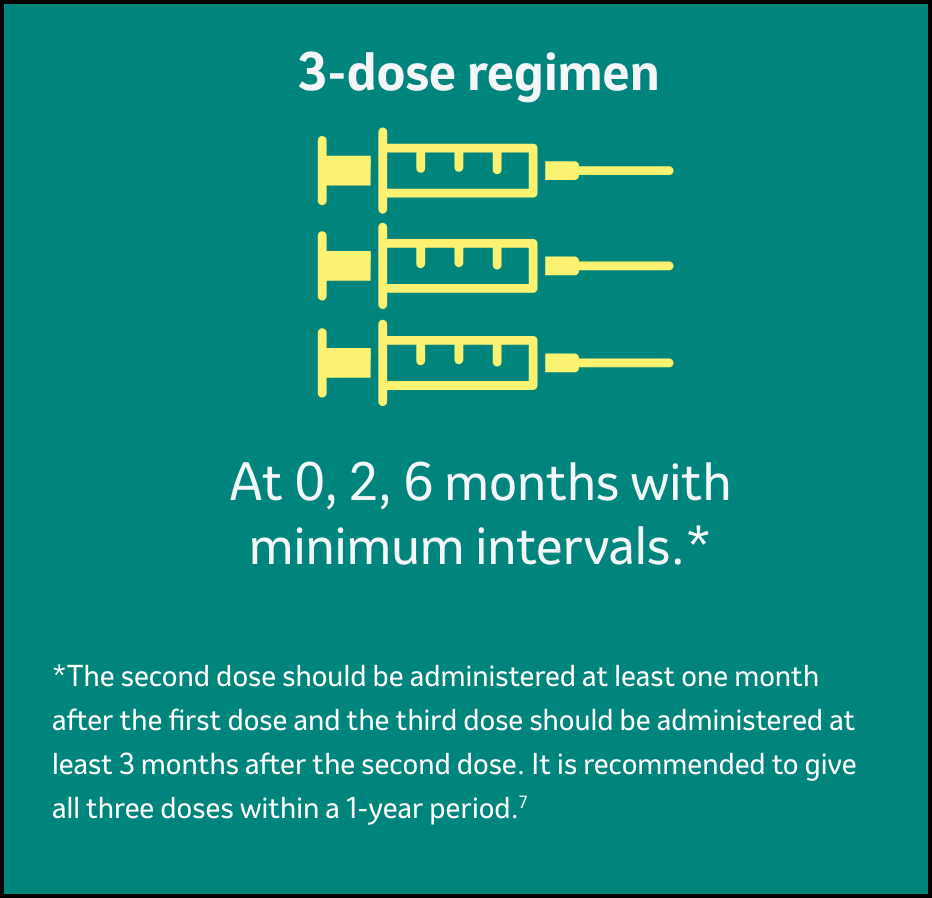
Dosing and administration for GARDASIL™47
Each dose of GARDASILTM4 is 0.5-mL, and it should be administered as shown below7:
Individuals 9-13 years of age
Individuals 14 years and older
Method of administration7:
- Do not dilute or mix GARDASILTM4 with other vaccines.
- Shake well immediately before use to maintain suspension of the vaccine.
-
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not use the product if particulates are present or if it appears discolored. After thorough agitation, GARDASILTM4 is a white cloudy liquid.
- Administer intramuscularly in the deltoid or anterolateral area of the thigh.
- Observe patients for 15 minutes after administration.
Abbreviations:
FDA = Food and Drug Administration
HCP = healthcare provider
HPV = human papillomavirus
REFERENCES
- Polonijo AN, Mahapatra D, Brown B. “I Thought It Was Just For Teenagers”: Knowledge, Attitudes, and Beliefs about HPV Vaccination Among Women Aged 27 to 45. Women’s Health Issues 2022;32-3:301–308.
- Leung SOA, Akinwunmi B, Elias KM, Feldman S. Educating healthcare providers to increase Human Papillomavirus (HPV) vaccination rates: A Qualitative Systematic Review. Vaccine X. 2019 Aug 5;3:100037.
- Alber JM, Askay D, Kolodziejski LR, Ghazvini S, Tolentino B, Gibbs SL. HPV Vaccine-Related Beliefs and Knowledge among Adults 18–45 Years Old. American Journal of Health Education, 2020;52(1):30-36.
- Alaraj RA, Brown B, Polonijo AN. “If People Were Told About the Cancer, They’d Want to Get Vaccinated”: Knowledge, Attitudes, and Beliefs About HPV Vaccination Among Mid-Adult Men. Am J Mens Health. 2023;17(1):15579883231153310.
- Austin B, Morgan H. Improving Human Papillomavirus Vaccine Uptake in the Family Practice Setting. The Journal for Nurse Practitioners 2019;15:e123-e125.
- Communicating with caregivers about the Human Papillomavirus vaccination: a tool to build confidence in communication skills among health workers. Copenhagen: WHO Regional Office for Europe; 2023. License: CC BY-NC-SA 3.0 IGO. In: World Health Organization. https://www.who.int/europe/publications/i/item/WHO-EURO-2023-6865-46631-67769. Accessed February 12, 2025.
- Gardasil SPC, Oct 2024
Selected Safety Information of Gardasil™
GARDASIL [HUMAN PAPILLOMAVIRUS QUADRIVALENT (TYPES 6,11,16,18) RECOMBINANT VACCINE]
Therapeutic indications
Gardasil is a vaccine for use from the age of 9 years for the prevention of:
- Premalignant genital lesions (cervical, vulvar and vaginal), premalignant anal lesions, cervical cancers and anal cancers causally related to certain oncogenic Human Papillomavirus (HPV) types.
- Genital warts (condyloma acuminata) causally related to specific HPV types.
The use of Gardasil should be in accordance with official recommendations.
Posology and method of administration
Posology
Individuals 9 to and including 13 years of age
Gardasil can be administered according to a 2-dose schedule (0.5 ml at 0, 6 months).
If the second vaccine dose is administered earlier than 6 months after the first dose, a third dose should always be administered.
Alternatively, Gardasil can be administered according to a 3-dose (0.5 ml at 0, 2, 6 months) schedule. The second dose should be administered at least one month after the first dose and the third dose should be administered at least 3 months after the second dose. All three doses should be given within a 1-year period.
Individuals 14 years of age and older
Gardasil should be administered according to a 3-dose (0.5 ml at 0, 2, 6 months) schedule.
The second dose should be administered at least one month after the first dose and the third dose should be administered at least 3 months after the second dose. All three doses should be given within a 1-year period.
The use of Gardasil should be in accordance with official recommendations.
Paediatric population
The safety and efficacy of Gardasil in children below 9 years of age have not been established. No data are available.
It is recommended that individuals who receive a first dose of Gardasil complete the vaccination course with Gardasil.
The need for a booster dose has not been established.
Method of administration
The vaccine should be administered by intramuscular injection. The preferred site is the deltoid area of the upper arm or in the higher anterolateral area of the thigh.
Gardasil must not be injected intravascularly. Neither subcutaneous nor intradermal administration has been studied. These methods of administration are not recommended.
Contraindications
- Hypersensitivity to the active substances or to any of the excipients.
- Individuals who develop symptoms indicative of hypersensitivity after receiving a dose of Gardasil should not receive further doses of Gardasil.
- Administration of Gardasil should be postponed in individuals suffering from an acute severe febrile illness. However, the presence of a minor infection, such as a mild upper respiratory tract infection or low-grade fever, is not a contraindication for immunisation.
Special warnings and precautions for use
Traceability
In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
The decision to vaccinate an individual should take into account the risk for previous HPV exposure and potential benefit from vaccination.
As with all injectable vaccines, appropriate medical treatment should always be readily available in case of rare anaphylactic reactions following the administration of the vaccine.
Syncope (fainting), sometimes associated with falling, can occur following, or even before, any vaccination, especially in adolescents as a psychogenic response to the needle injection. This can be accompanied by several neurological signs such as transient visual disturbance, paraesthesia, and tonic-clonic limb movements during recovery. Therefore, vaccinees should be observed for approximately 15 minutes after vaccine administration. It is important that procedures are in place to avoid injury from faints.
As with any vaccine, vaccination with Gardasil may not result in protection in all vaccine recipients.
Gardasil will only protect against diseases that are caused by HPV types 6, 11, 16 and 18 and to a limited extent against diseases caused by certain related HPV types. Therefore, appropriate precautions against sexually transmitted diseases should continue to be used.
Gardasil is for prophylactic use only and has no effect on active HPV infections or established clinical disease. Gardasil has not been shown to have a therapeutic effect. The vaccine is therefore, not indicated for treatment of cervical cancer, high-grade cervical, vulvar, and vaginal dysplastic lesions or genital warts. It is also not intended to prevent progression of other established HPV-related lesions.
Gardasil does not prevent lesions due to a vaccine HPV type in individuals infected with that HPV type at the time of vaccination.
The use of Gardasil in adult women should take into consideration the variability of HPV type prevalence in different geographical areas.
Vaccination is not a substitute for routine cervical screening. Since no vaccine is 100 % effective and Gardasil will not provide protection against every HPV type, or against existing HPV infections, routine cervical screening remains critically important and should follow local recommendations.
Safety and immunogenicity of the vaccine have been assessed in individuals aged from 7 to 12 years who are known to be infected with human immunodeficiency virus (HIV).
Individuals with impaired immune responsiveness, due to either the use of potent immunosuppressive therapy, a genetic defect, or other causes, may not respond to the vaccine.
This vaccine should be given with caution to individuals with thrombocytopaenia or any coagulation disorder because bleeding may occur following an intramuscular administration in these individuals.
Long-term follow-up studies were conducted to determine the duration of protection.
There are no safety, immunogenicity or efficacy data to support change during vaccination with Gardasil to other HPV vaccines which do not cover the same HPV types. Therefore, it is important that the same vaccine should be prescribed for the whole dose regimen.
Sodium
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, that is to say essentially ‘sodium-free’.
Interaction with other medicinal products and other forms of interaction
In all clinical trials, individuals who had received immunoglobulin or blood-derived products during the 6 months prior to the first vaccine dose were excluded.
Use with other vaccines
Administration of Gardasil at the same time (but, for injected vaccines, at a different injection site) as hepatitis B (recombinant) vaccine did not interfere with the immune response to the HPV types. The seroprotection rates (proportion of individuals reaching seroprotective level anti-HBs >10 mIU/ml) were unaffected (96.5 % for concomitant vaccination and 97.5 % for hepatitis B vaccine only). Anti-HBs geometric mean antibody titres were lower on co-administration, but the clinical significance of this observation is not known.
Gardasil may be administered concomitantly with a combined booster vaccine containing diphtheria (d)and tetanus (T) with either pertussis [acellular, component] (ap) and/or poliomyelitis [inactivated] (IPV) (dTap, dT-IPV, dTap-IPV vaccines) with no significant interference with antibody response to any of the components of either vaccine. However, a trend of lower anti-HPV GMTs was observed in the concomitant group. The clinical significance of this observation is not known. This is based on the results from a clinical trial in which a combined dTap-IPV vaccine was administered concomitantly with the first dose of Gardasil.
The concomitant administration of Gardasil with vaccines other than the ones above has not been studied.
Use with hormonal contraceptives
In clinical studies, 57.5 % of women aged 16 to 26 years and 31.2 % of women aged 24 to 45 years who received Gardasil used hormonal contraceptives during the vaccination period. Use of hormonal contraceptives did not appear to affect the immune response to Gardasil.
Fertility, pregnancy and lactation
Pregnancy
Specific studies of the vaccine in pregnant women were not conducted. During the clinical development program, 3,819 women (vaccine = 1,894 vs. placebo = 1,925) reported at least one pregnancy. There were no significant differences in types of anomalies or proportion of pregnancies with an adverse outcome in Gardasil and placebo treated individuals. These data on pregnant women (more than 1,000 exposed outcomes) indicate no malformative nor feto/ neonatal toxicity.
The data on Gardasil administered during pregnancy did not indicate any safety signal. However, these data are insufficient to recommend use of Gardasil during pregnancy. Vaccination should be postponed until completion of pregnancy.
Breast-feeding
In breast-feeding mothers given Gardasil or placebo during the vaccination period of the clinical trials the rates of adverse reactions in the mother and the breast-fed infant were comparable between the vaccination and the placebo groups. In addition, vaccine immunogenicity was comparable among breast-feeding mothers and women who did not breast-feed during the vaccine administration.
Therefore, Gardasil can be used during breast-feeding.
Fertility
Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. No effects on male fertility were observed in rats.
Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
Undesirable effects
A. Summary of the safety profile
In 7 clinical trials (6 placebo-controlled), individuals were administered Gardasil or placebo on the day of enrolment and approximately 2 and 6 months thereafter. Few individuals (0.2 %) discontinued due to adverse reactions. Safety was evaluated in either the entire study population (6 studies) or in a predefined subset (one study) of the study population using vaccination report card (VRC)-aided surveillance for 14 days after each injection of Gardasil or placebo. The individuals who were monitored using VRC-aided surveillance included 10,088 individuals (6,995 females 9 to 45 years of age and 3,093 males 9 to 26 years of age at enrolment) who received Gardasil and 7,995 individuals (5,692 females and 2,303 males) who received placebo. The most common adverse reactions observed were injection-site adverse reactions (77.1 % of vaccinees within 5 days following any vaccination visit) and headache (16.6 % of the vaccinees). These adverse reactions usually were mild or moderate in intensity.
B. Tabulated summary of adverse reactions
Clinical Trials
Table 1 presents vaccine-related adverse reactions which were observed among recipients of Gardasil at a frequency of at least 1.0 % and also at a greater frequency than observed among placebo recipients. They are ranked under headings of frequency using the following convention:
[Very Common (≥1/10); Common (≥1/100 to <1/10); Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000); Very Rare (<1/10,000)]
Post-Marketing Experience
Table 1 also includes additional adverse events which have been spontaneously reported during the post-marketing use of Gardasil worldwide. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Consequently, the frequency of these adverse events is qualified as “not known”.
Table 1: Adverse Events Following Administration of Gardasil from Clinical Trials and Post-Marketing Surveillance

*Post Marketing adverse events (frequency cannot be estimated from the available data).
1 During clinical trials, dizziness was observed as a common adverse reaction in females. In males, dizziness was not observed at a greater frequency in vaccine recipients than in placebo recipients.
In addition, in clinical trials adverse reactions that were judged to be vaccine- or placebo-related by the study investigator were observed at frequencies lower than 1 %:
Respiratory, thoracic and mediastinal disorders:
Very rare: bronchospasm.
Skin and subcutaneous tissue disorders:
Rare: urticaria.
Nine cases (0.06 %) of urticaria were reported in the Gardasil group and 20 cases (0.15 %) were seen in the adjuvant-containing placebo group.
In the clinical studies, individuals in the Safety Population reported any new medical conditions during the follow-up. Among 15,706 individuals who received Gardasil and 13,617 individuals who received placebo, there were 39 cases of non-specific arthritis/arthropathy reported, 24 in the Gardasil group and 15 in the placebo group.
In a clinical trial of 843 healthy adolescent males and females 11-17 years of age, administration of the first dose of Gardasil concomitantly with a combined diphtheria, tetanus, pertussis [acellular, component] and poliomyelitis [inactivated] booster vaccine showed that there was more injection-site swelling and headache reported following concomitant administration. The differences observed were < 10 % and in the majority of subjects, the adverse events were reported as mild to moderate in intensity.
Overdose
There have been reports of administration of higher than recommended doses of Gardasil. In general, the adverse event profile reported with overdose was comparable to recommended single doses of Gardasil.
SA-HPV-00072 | Expiry Date: 02-12-2026
