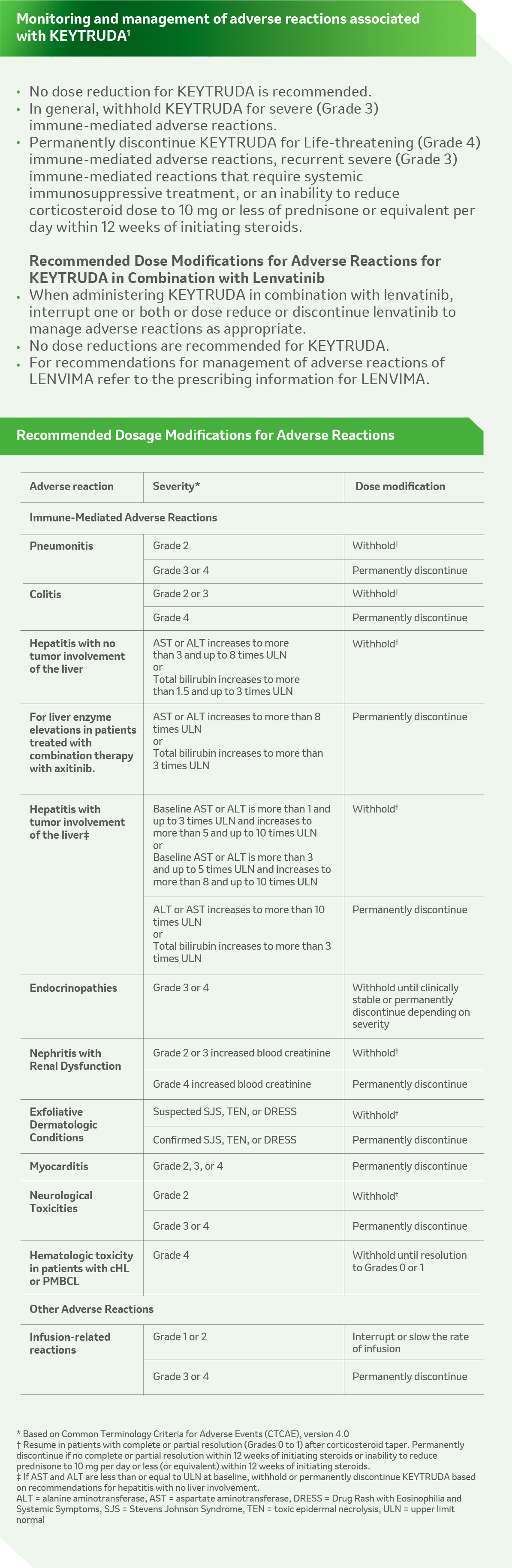
KN 755 – adverse events management


Reference:
- KEYTRUDA USPI.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) forUterine Neoplasms V.1.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. To view themost recent and complete version of the guidelines, go online to NCCN. org. Available at:https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
- Bartley AN, Hamilton SR, Alsabeh R, et al. Template for reporting results of biomarker testing of specimensfrom patients with carcinoma of the colon and rectum. Arch Pathol Lab Med. 2014;138(2):166–170.
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol.2010;7(3):153–162
- Dudley JC, Lin M-T, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res.2016;22(4):813-820
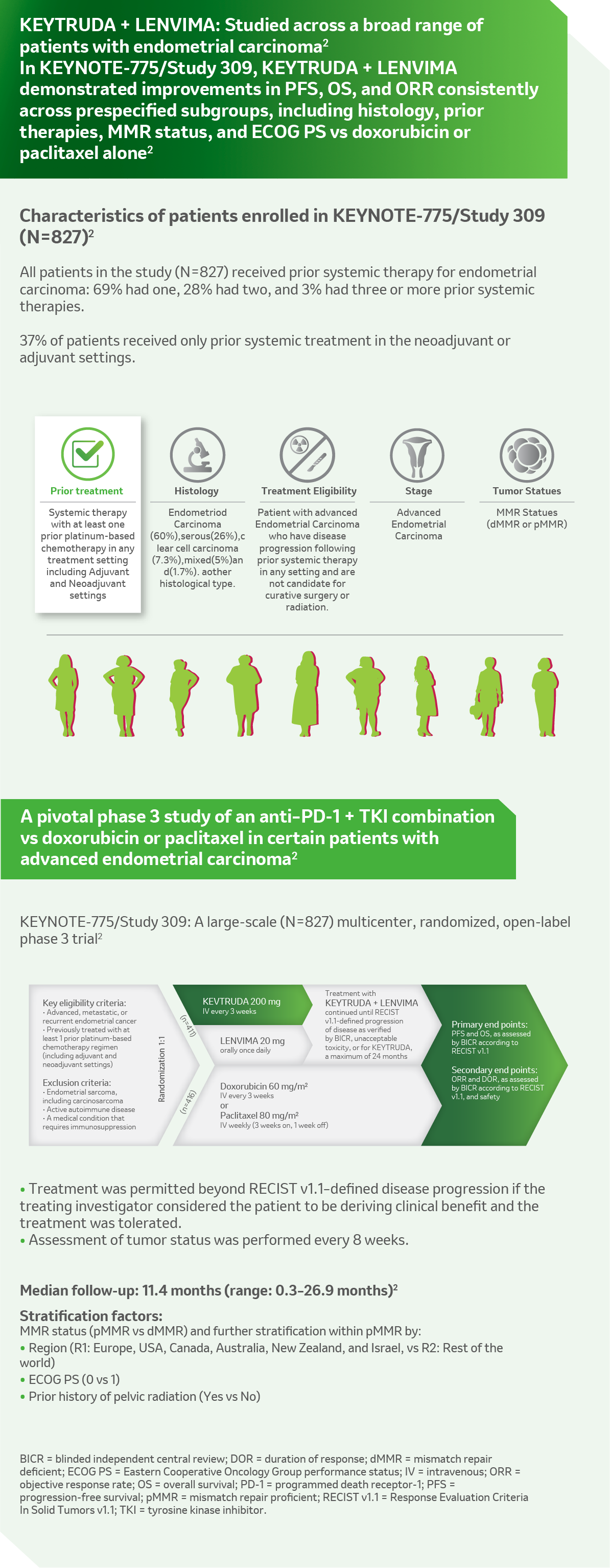
Reference:
- KEYTRUDA USPI.
- Makker V, Colombo N, Herráez AC, et al. A multicenter, open-label, randomized, phase 3 study to compare theefficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patientswith advanced endometrial cancer: Study 309/KEYNOTE-775. Slide deck presented at: Virtual AnnualMeeting on Women’s Cancer; 19–25 March 2021
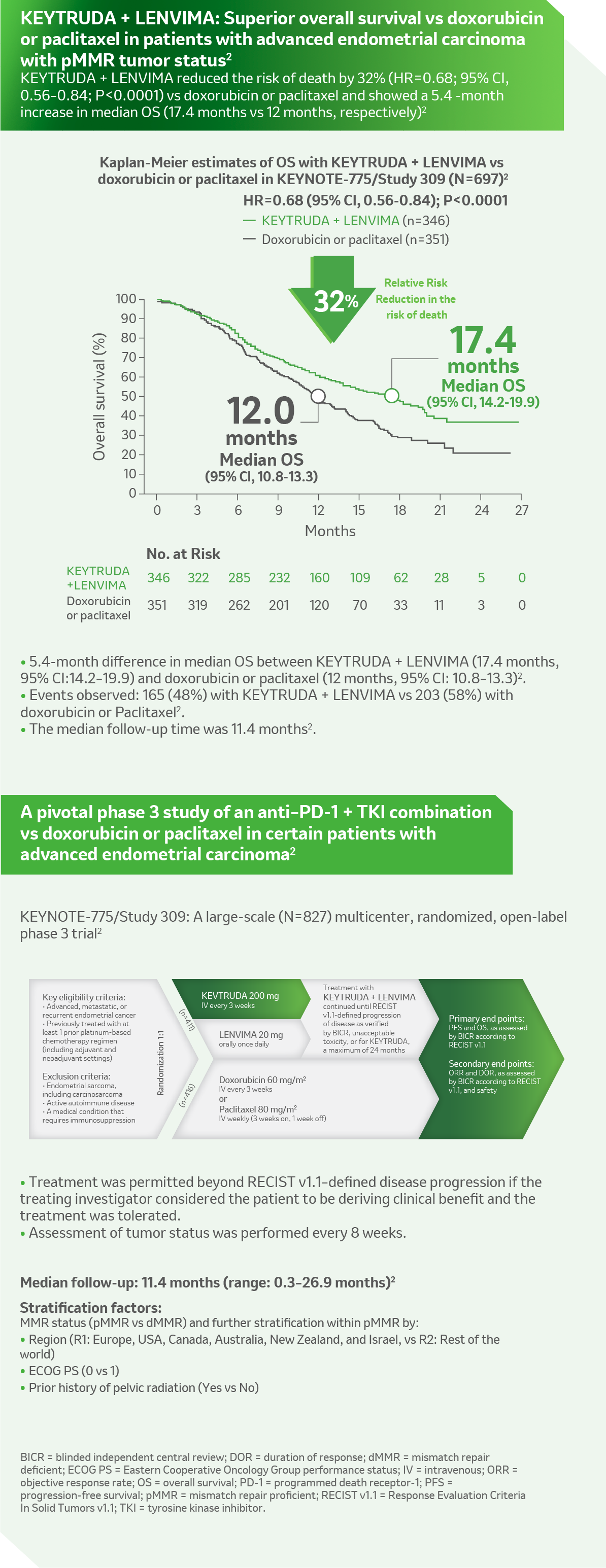
Reference:
- KEYTRUDA USPI.
- Makker V, Colombo N, Herráez AC, et al. A multicenter, open-label, randomized, phase 3 study to compare theefficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patientswith advanced endometrial cancer: Study 309/KEYNOTE-775. Slide deck presented at: Virtual AnnualMeeting on Women’s Cancer; 19–25 March 2021

Reference:
- KEYTRUDA USPI.
- Makker V, Colombo N, Herráez AC, et al. A multicenter, open-label, randomized, phase 3 study to compare theefficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patientswith advanced endometrial cancer: Study 309/KEYNOTE-775. Slide deck presented at: Virtual AnnualMeeting on Women’s Cancer; 19–25 March 2021

Reference:
- KEYTRUDA USPI.
- Makker V, Colombo N, Herráez AC, et al. A multicenter, open-label, randomized, phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer: Study 309/KEYNOTE-775. Slide deck presented at: Virtual Annual Meeting on Women’s Cancer; 19–25 March 2021
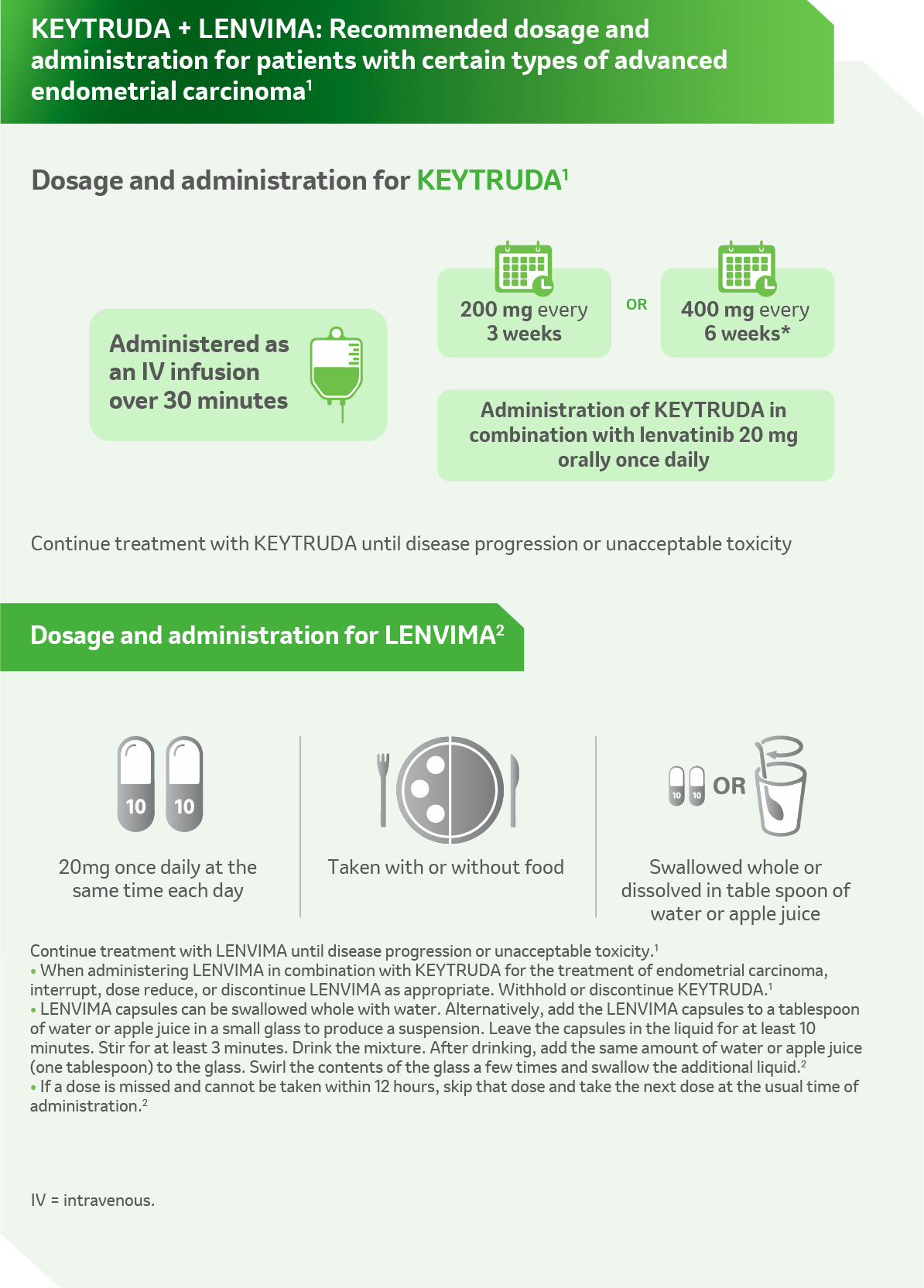
Reference:
- KEYTRUDA USPI.
- LENVIMA Core Data sheet
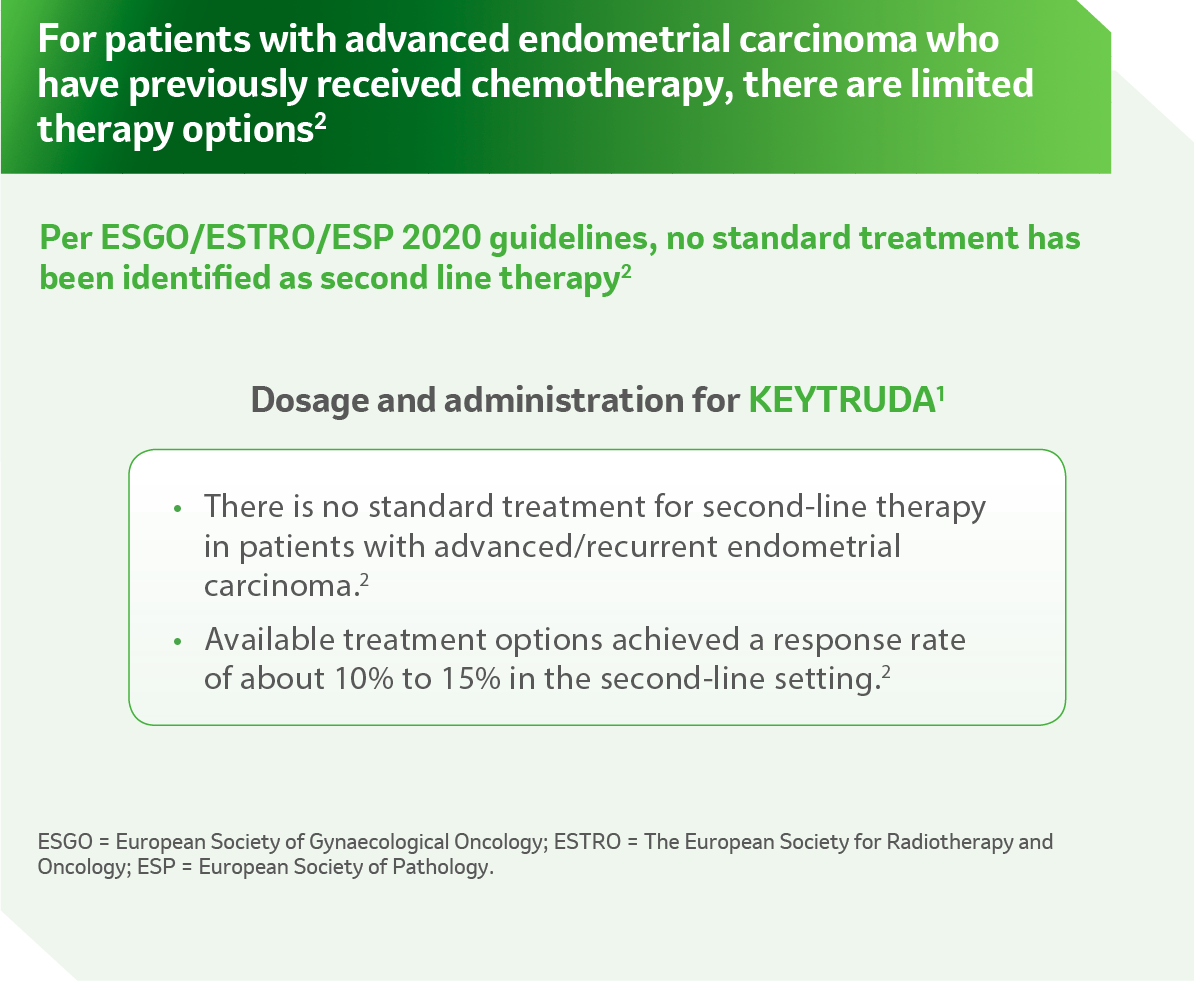
Reference:
- KEYTRUDA USPI.
- Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients withendometrial carcinoma. Int J Gynecol Cancer. Published online December 18, 2020. doi:10.1136/ijgc-2020- 002230.
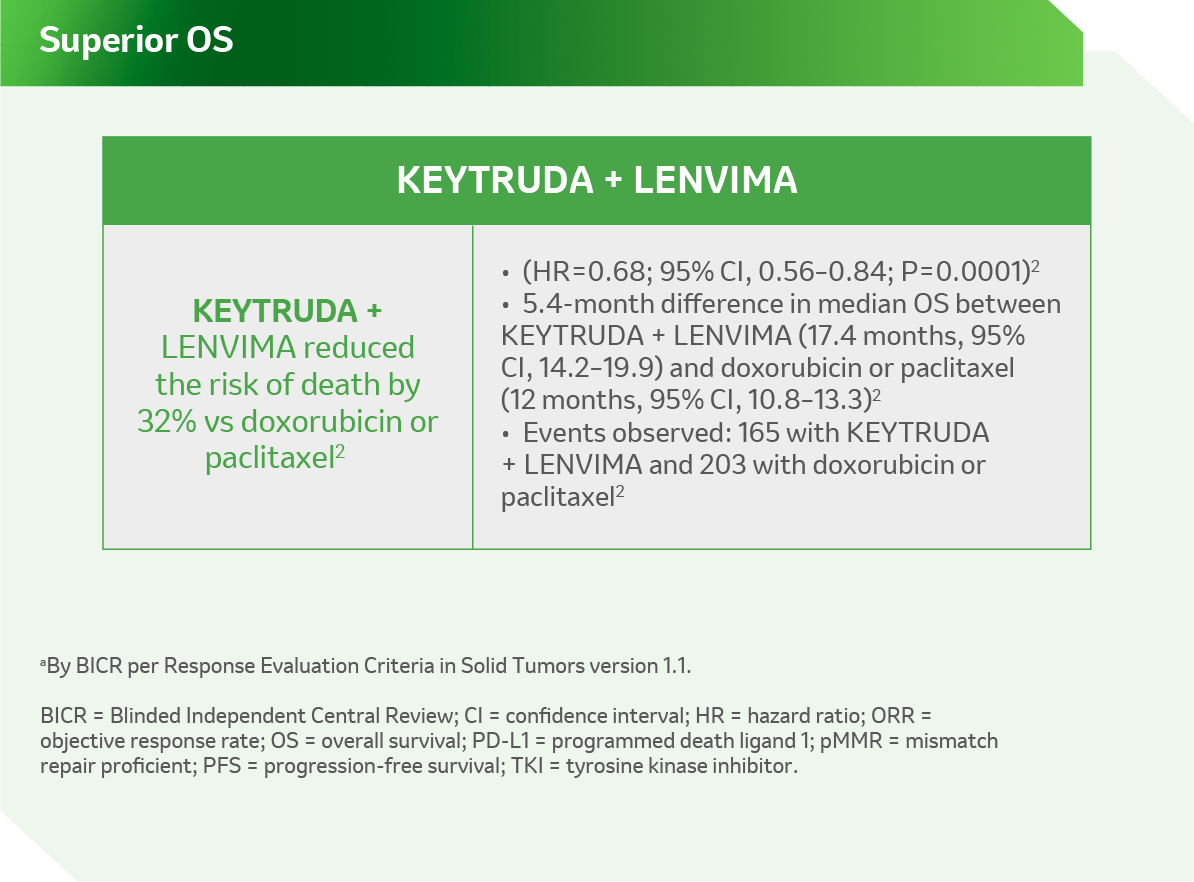
Reference:
- KEYTRUDA USPI.
- Makker V, Colombo N, Herráez AC, et al. A multicenter, open-label, randomized, phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer: Study 309/KEYNOTE-775. Slide deck presented at: Virtual Annual Meeting on Women’s Cancer; 19–25 March 2021
AE-END-00004 Exp 30 MAY 2025
AE-END-00002 | Exp 20 FEB 2025
BH-END-00001 | Exp 15 FEB 2025