KN-811


References:
- KEYTRUDA® Summary of Product Characteristics
- US Food and Drug Administration. FDA amends pembrolizumab’s gastric cancer indication. Available at: https://www.fda.gov/drugs/ resources-information-approved-drugs/fda-amendspembrolizumabsgastric- cancer-indication. Last accessed: 20-2-2025.
- Jemperli Summary of Product Characteristics
- Libtayo Summary of Product Characteristics
- Opdivo Summary of Product Characteristics
- Opdualag Summary of Product Characteristics
- Marieb EN, Hoehn K. The digestive system. In Marieb EN, Hoehn N, eds. Human Anatomy & Physiology. 11th ed. Pearson; 2019868-925.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0Epub 2023 Oct 20. PMID: 37871604. supplementary appendix
References:
- KEYTRUDA® Summary of Product Characteristics.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) for Gastric Cancer Version 5.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. last Access date:13/2/2025.
References:
- KEYTRUDA® Summary of Product Characteristics
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) for Gastric Cancer VERSION 5.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. last Access date:14/2/2025.
- Symptoms of advanced stomach cancer. Cancer Research UK. Updated 17 October 2022. Accessed 13 February 2025. https://www.cancerresearchuk.org/aboutcancer/ stomach-cancer/ advancedcancer/symptoms-advanced-cancer
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005-1020. doi:10.1016/j.annonc. Access date: 14/2/2025.
- US Food and Drug Administration. FDA amends pembrolizumab’s gastric cancer indication. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amendspembrolizumabsgastric- cancer-indication. Last accessed: 14-2-2025.
- Jemperli Summary of Product Characteristics
- Libtayo Summary of Product Characteristics
- Opdivo Summary of Product Characteristics
- Opdualag Summary of Product Characteristics
- Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E, Tabernero J, Luo S, MahaveM, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi:10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
- Supplementary appendix of Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E,Tabernero J, Luo S, Mahave M, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi: 10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0Epub 2023 Oct 20. PMID: 37871604.supplementary appendix.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.

References:
- KEYTRUDA® Summary of Product Characteristics
- US Food and Drug Administration. FDA amends pembrolizumab’s gastric cancer indication. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amendspembrolizumabsgastric- cancer-indication. Last accessed: 14-2-2025.
- Jemperli Summary of Product Characteristics
- Libtayo Summary of Product Characteristics
- Opdivo Summary of Product Characteristics
- Opdualag Summary of Product Characteristics
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0Epub 2023 Oct 20. PMID: 37871604.supplementary appendix.
References:
- KEYTRUDA® Summary of Product Characteristics
- US Food and Drug Administration. FDA amends pembrolizumab’s gastric cancer indication. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amendspembrolizumabsgastric- cancer-indication. Last accessed: 14-02-2025.
- Jemperli Summary of Product Characteristics
- Libtayo Summary of Product Characteristics
- Opdivo Summary of Product Characteristics
- Opdualag Summary of Product Characteristics
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0Epub 2023 Oct 20. PMID: 37871604.supplementary appendix.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.

References:
- KEYTRUDA® Summary of Product Characteristics
- US Food and Drug Administration. FDA amends pembrolizumab’s gastric cancer indication. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amendspembrolizumabsgastric- cancer-indication. Last accessed: 14-02-2025.
- Jemperli Summary of Product Characteristics
- Libtayo Summary of Product Characteristics
- Opdivo Summary of Product Characteristics
- Opdualag Summary of Product Characteristics
- Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E, Tabernero J, Luo S, Mahave M, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi:10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
- Supplementary appendix of Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E,Tabernero J, Luo S, Mahave M, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi: 10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0 Epub 2023 Oct 20. PMID: 37871604.supplementary appendix.

References:
- KEYTRUDA® Summary of Product Characteristics
- US Food and Drug Administration. FDA amends pembrolizumab’s gastric cancer indication. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amendspembrolizumabsgastric- cancer-indication. Last accessed: 14-02-2025.
- Jemperli Summary of Product Characteristics
- Libtayo Summary of Product Characteristics
- Opdivo Summary of Product Characteristics
- Opdualag Summary of Product Characteristics
- Supplementary appendix of Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E,Tabernero J, Luo S, Mahave M, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi: 10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0Epub 2023 Oct 20. PMID: 37871604.supplementary appendix.
- Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.
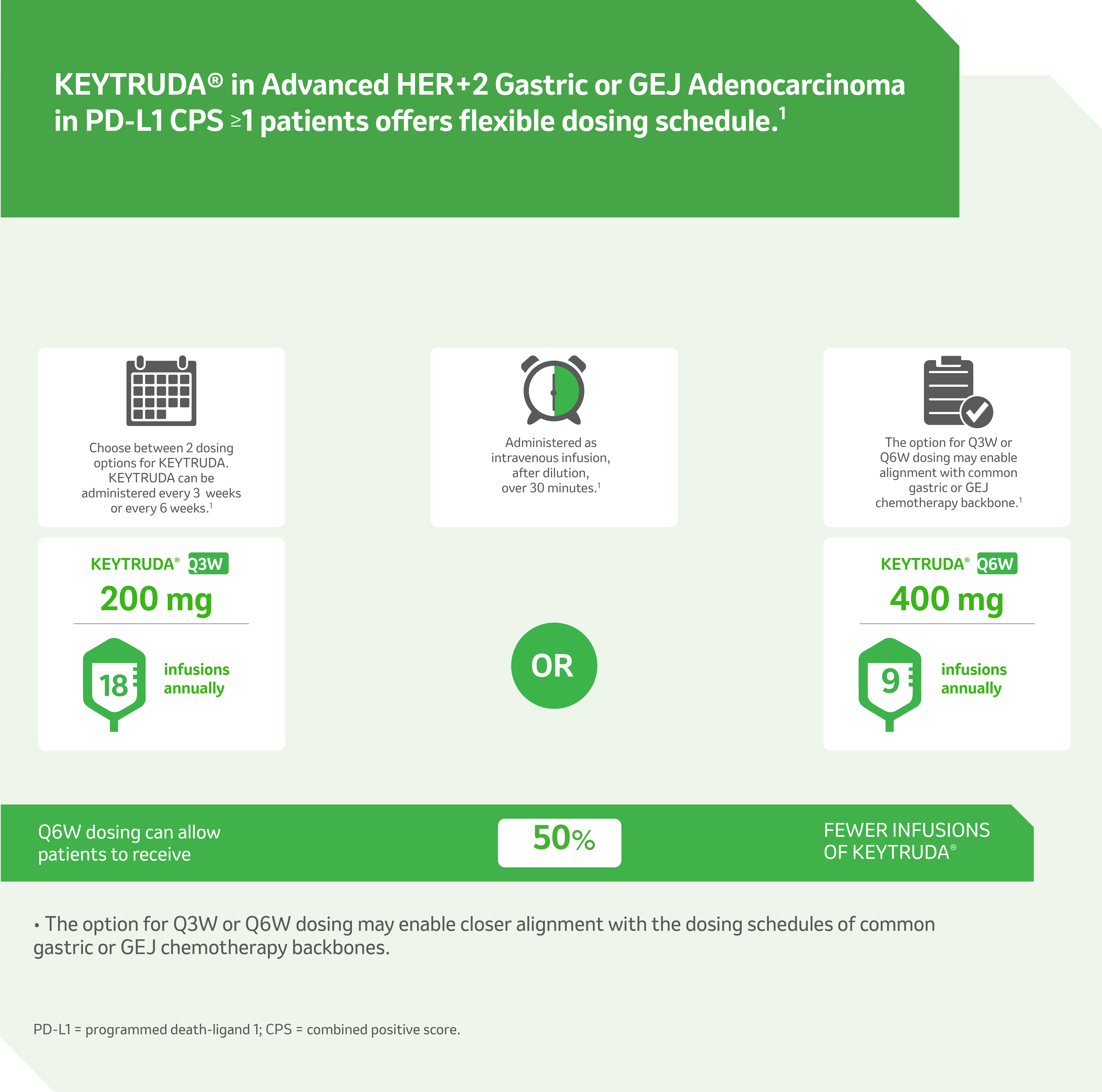
References:
- KEYTRUDA® Summary of Product Characteristics


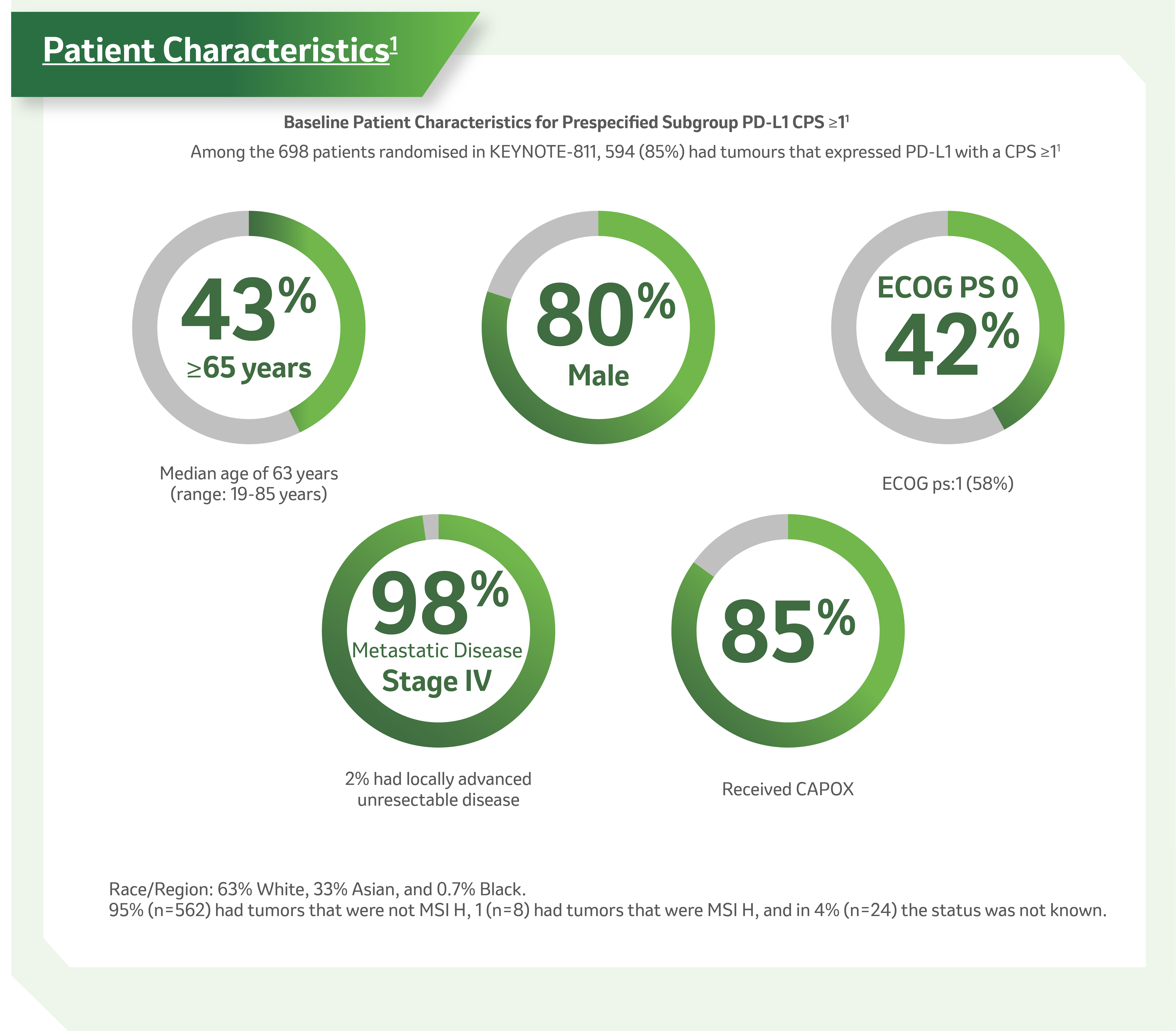
References:
1. KEYTRUDA® Summary of Product Characteristics.
2. Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E, Tabernero J, Luo S, MahaveM, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi:10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
3. Supplementary appendix of Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yañez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin S, Van Cutsem E,Tabernero J, Luo S, Mahave M, Tang Y, Lowery M, Monteiro MMF, Xu L, Shih CS, Sharan KP, Bhagia P, Rha SY. Pembrolizumab in HER2-Positive Gastric Cancer. N Engl J Med. 2024 Oct 10;391(14):1360-1362. doi: 10.1056/NEJMc2408121. Epub 2024 Sep 14. PMID: 39282917.
4. Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0Epub 2023 Oct 20. PMID: 37871604.supplementary appendix.
5. Janjigian YY, Kawazoe A, Bai Y, KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo controlled trial. Lancet. 2023 Dec 9;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0. Epub 2023 Oct 20. PMID: 37871604.
SA-KEY-00550 | Exp 20 Feb 2027.