Recommending HPV Vaccine-test
HCP (health care provider) recommendation is a key determinant of HPV vaccine uptake1

High-quality recommendations by physicians can increase vaccination series initiation by 3-fold and completion by 9-fold.2
Your recommendation is significantly related to both intention to receive the vaccine and to receiving a full dose of the vaccine among those who had not been vaccinated (p<0.001).3
There is a large knowledge gap on eligible vaccination age groups3
Just as it is beneficial to discuss vaccination with the parents of children who are in the recommended age group, there is a need to encourage provider conversation with adults who can benefit from vaccination.3

An online survey conducted among 421 US adults 18-45 years old in April 2019 found 96 % of participants were unaware that the HPV vaccine had been approved for adults.3
Only 4 % of those surveyed were aware of the 2018 FDA (Food and Drug Administration) changes about approval for adults 26–45 years.3
Survey results3
30 %
Received provider recommendation for the HPV vaccine

35 %
Provider started a conversation about the HPV vaccine in general

26.4 %
Received at least one dose of the HPV vaccine

19.5 %
Received the full three doses

A survey study was conducted to examine knowledge about changes to the FDA’s approval of the HPV vaccine for adults ages 26-45 years old and to explore which IBM constructs are significantly related to HPV vaccine intention. A sample was recruited through a national paneling company. 420 participants were included in the study. The inclusion criteria were being between 18-45 years old and currently living in the United States. Eligible participants completed a 15-minute study administered through software. Attitudes, perceived norms, and perceived control were measured on 5-point Likert scales (1=strongly disagree, 5=strongly agree), and knowledge questions were developed to examine a participant’s knowledge of the number of doses required per age group and currently approved age groups by the FDA. Averages across items were calculated to create composite scores for each construct.3
Changing patient perspectives on HPV vaccines

“I trust my doctor and his group to tell me what I should know.”4
Male participants in a 2023 qualitative study noted that they considered their HCPs to be trusted sources of HPV vaccine information.4
Despite being preferred sources of HPV vaccine information among the men in this study, most participants had never discussed HPV vaccination with an HCP.4
Two main reasons why HPV vaccination was not discussed with a healthcare provider4:

HCP recommendations tend to be less frequent and of lower quality when the patient is male

Many U.S. physicians are unaware of the new shared clinical decision-making guidance for adults aged 27-45

Nurse practitioners also play a role in HPV vaccine recommendations!5
Nurses are at an advantage in the clinical setting to narrow the gap between education and vaccine intention.5
Key points to include in conversation with patients about HPV vaccination6

Explain when to vaccinate and why the HPV vaccine is important

Proactively explain potential side-effects of the HPV vaccine

Promote safety of the HPV vaccine

Focus on cancer prevention

Emphasize long lasting protection
Keep in mind that the long-term goal for these conversations is vaccine acceptance, and it may take more than one visit to win your patient’s confidence.6
Dosing and administration for GARDASIL®97
Each dose of GARDASIL®9 is 0.5-mL, and it should be administered as shown below7:
Individuals 9-14 years of age
2-dose regimen
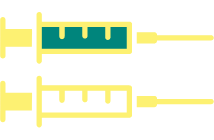
At 0, 6-12 months*
Individuals 15-45 years of age
3-dose regimen
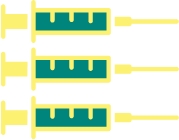
At 0, 2, 6 months
*If the second dose is administered earlier than 5 months after the first dose, administer a third dose at least 4 months after the second dose.7
Method of administration7:
- Do not dilute or mix GARDASIL®9 with other vaccines.
- Shake well immediately before use to maintain suspension of the vaccine.
-
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not use the product if particulates are present or if it appears discolored. After thorough agitation, GARDASIL®9 is a white cloudy liquid.
- Administer intramuscularly in the deltoid or anterolateral area of the thigh.
- Observe patients for 15 minutes after administration.
Abbreviations:
FDA = Food and Drug Administration
HCP = healthcare provider
HPV = human papillomavirus
REFERENCES
- Polonijo AN, Mahapatra D, Brown B. “I Thought It Was Just For Teenagers”: Knowledge, Attitudes, and Beliefs about HPV Vaccination Among Women Aged 27 to 45. Women’s Health Issues 2022;32-3:301–308.
- Leung SOA, Akinwunmi B, Elias KM, Feldman S. Educating healthcare providers to increase Human Papillomavirus (HPV) vaccination rates: A Qualitative Systematic Review. Vaccine X. 2019 Aug 5;3:100037.
- Alber JM, Askay D, Kolodziejski LR, Ghazvini S, Tolentino B, Gibbs SL. HPV Vaccine-Related Beliefs and Knowledge among Adults 18–45 Years Old. American Journal of Health Education, 2020;52(1):30-36.
- Alaraj RA, Brown B, Polonijo AN. “If People Were Told About the Cancer, They’d Want to Get Vaccinated”: Knowledge, Attitudes, and Beliefs About HPV Vaccination Among Mid-Adult Men. Am J Mens Health. 2023;17(1):15579883231153310.
- Austin B, Morgan H. Improving Human Papillomavirus Vaccine Uptake in the Family Practice Setting. The Journal for Nurse Practitioners 2019;15:e123-e125.
- Communicating with caregivers about the Human Papillomavirus vaccination: a tool to build confidence in communication skills among health workers. Copenhagen: WHO Regional Office for Europe; 2023. License: CC BY-NC-SA 3.0 IGO. In: World Health Organization. https://www.who.int/europe/publications/i/item/WHO-EURO-2023-6865-46631-67769. Accessed March 06, 2025.
- Gardasil 9 Prescribing Information.
More Related Information

GARDASIL™9
(HUMAN PAPILLOMAVIRUS 9-VALENT VACCINE, RECOMBINANT)
Suspension for intramuscular injection
Indications and usage
Girls and women
GARDASIL™9 is a vaccine indicated in girls and women 9 through 45 years of age for the prevention of the following diseases:
- Cervical, vulvar, vaginal, anal, oropharyngeal and other head and neck cancers caused by Human Papillomavirus (HPV) types 16, 18, 31, 33, 45, 52, and 58
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58:
- Cervical intraepithelial neoplasia (CIN) grade 2/3 and cervical adenocarcinoma in situ (AIS)
- Cervical intraepithelial neoplasia (CIN) grade 1
- Vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3
- Vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3
Boys and Men
GARDASIL 9 is indicated in boys and men 9 through 45 years of age for the prevention of the following diseases:
- Anal, oropharyngeal and other head and neck cancers caused by HPV types 16, 18, 31, 33,45, 52, and 58
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58:
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3
The oropharyngeal and head and neck cancer indication is approved under accelerated approval based on effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Limitations Of Use And Effectiveness
Vaccination with GARDASIL 9 does not eliminate the necessity for vaccine recipients to undergo screening for cervical, vulvar, vaginal, anal, oropharyngeal and other head and neck cancers as recommended by a health care provider.
- GARDASIL 9 has not been demonstrated to provide protection against disease caused by:
- HPV types not covered by the vaccine,
- HPV types to which a person has previously been exposed through sexual activity.
- Not all vulvar, vaginal, anal, oropharyngeal and other head and neck cancers are caused by HPV, and GARDASIL 9 protects only against those vulvar, vaginal, anal, oropharyngeal and other head and neck cancers caused by HPV 16, 18, 31, 33, 45, 52, and 58.
- GARDASIL 9 is not a treatment for external genital lesions; cervical, vulvar, vaginal, anal, oropharyngeal and other head and neck cancers; CIN; VIN; VaIN; or AIN.
- Vaccination with GARDASIL 9 may not result in protection in all vaccine recipients.
Dosage and administration (For intramuscular use only)
Dosage
Each dose of GARDASIL 9 is 0.5-mL.
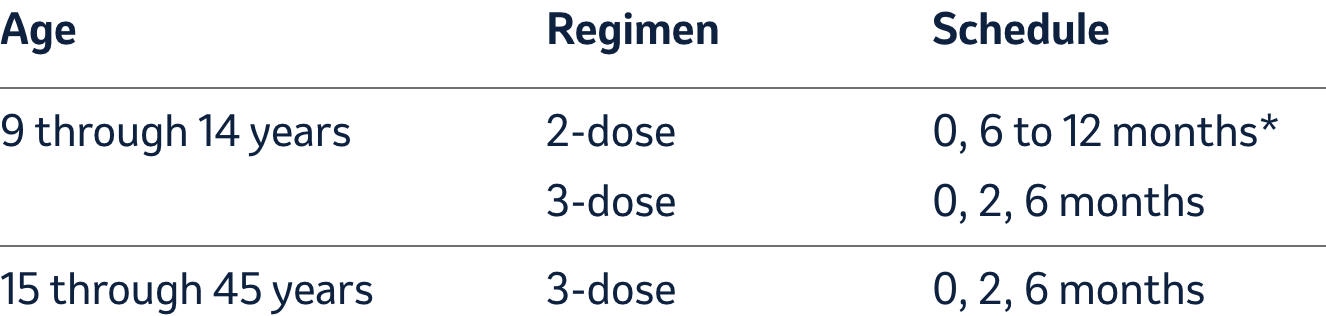
*If the second dose is administered earlier than 5 months after the first
dose, administer a third dose at least 4 months after the second dose.
Method of Administration
- Do not dilute or mix GARDASIL 9 with other vaccines.
- Shake well immediately before use to maintain suspension of the vaccine.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the product if particulates are present or if it appears discolored. After thorough agitation, GARDASIL 9 is a white cloudy liquid.
- Administer intramuscularly in the deltoid or anterolateral area of the thigh.
- Observe patients for 15 minutes after administration.
Single-Dose Vial Use
Withdraw the 0.5-mL dose of vaccine from the single-dose vial using a sterile needle and syringe and use promptly. Discard vial after use.
Prefilled Syringe Use
This package contains one needle. Shake well before use. Attach a needle by twisting in a clockwise direction until the needle fits securely on the syringe. Administer the entire dose as per standard protocol. Discard syringe after use.
Contraindications
Hypersensitivity, including severe allergic reactions to yeast (a vaccine component), or after a previous dose of GARDASIL 9 or GARDASIL
Warnings and precautions
Syncope
Because vaccinees may develop syncope, sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended. Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following HPV vaccination. When syncope is associated with tonic-clonic movements, the activity is usually transient and typically responds to restoring cerebral perfusion by maintaining a supine or Trendelenburg position
Managing Allergic Reactions
Appropriate medical treatment and supervision must be readily available in case of anaphylactic reactions following the administration of GARDASIL 9.
Drug interactions
Use with Systemic Immunosuppressive Medications
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune responses to vaccines
Description
Each 0.5-mL dose contains approximately 30 mcg of HPV Type 6 L1 protein, 40 mcg of HPV Type 11 L1 protein, 60 mcg of HPV Type 16 L1 protein, 40 mcg of HPV Type 18 L1 protein, 20 mcg of HPV Type 31 L1 protein, 20 mcg of HPV Type 33 L1 protein, 20 mcg of HPV Type 45 L1 protein, 20 mcg of HPV Type 52 L1 protein, and 20 mcg of HPV Type 58 L1 protein.
Each 0.5-mL dose of the vaccine also contains approximately 500 mcg of aluminum (provided as AAHS), 9.56 mg of sodium chloride, 0.78 mg of L-histidine, 50 mcg of polysorbate 80, 35 mcg of sodium borate, <7 mcg yeast protein, and water for injection. The product does not contain a preservative or antibiotics.
After thorough agitation, GARDASIL 9 is a white, cloudy liquid
Adverse reactions
Injection-Site and Systemic Adverse Reactions
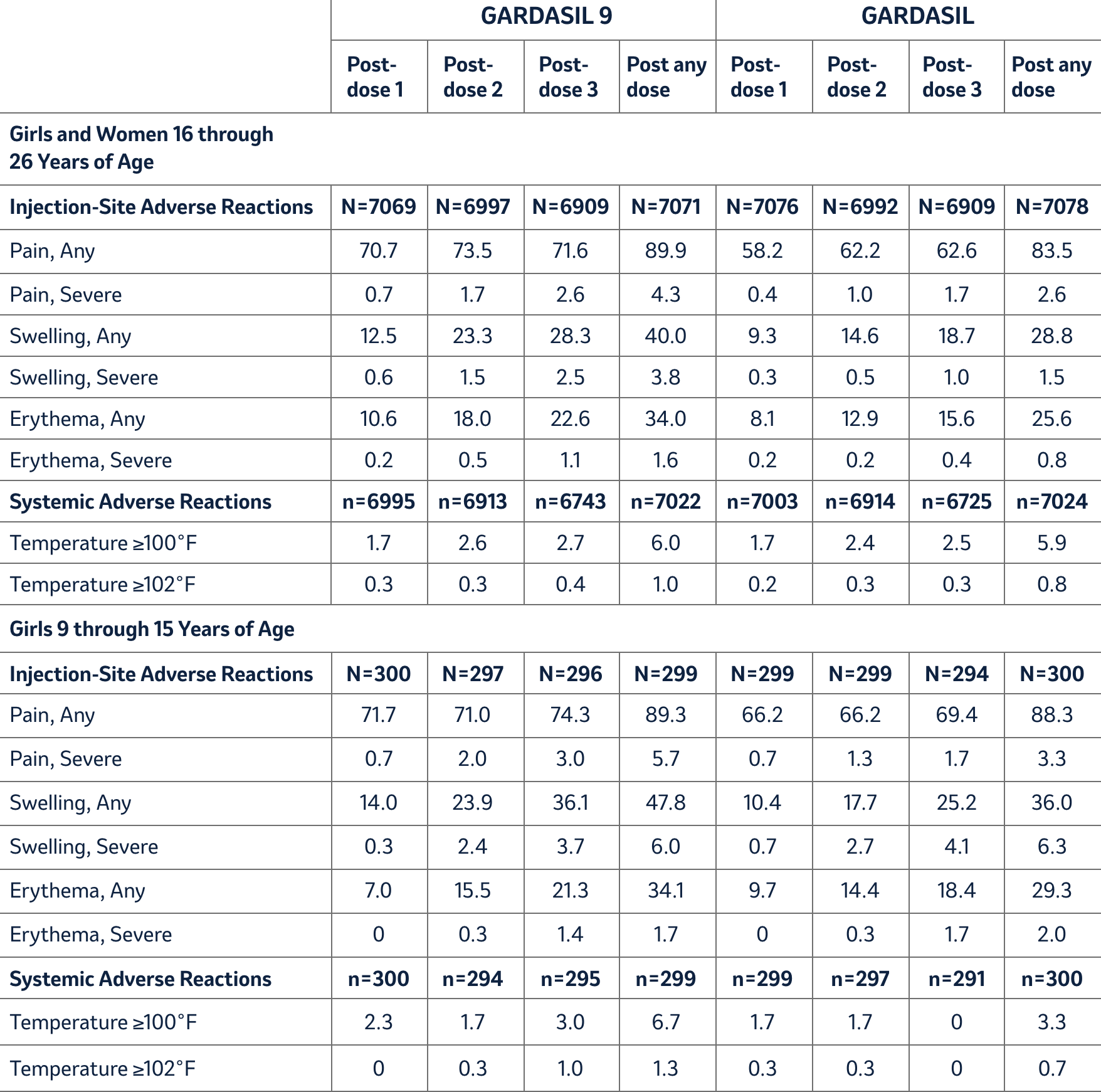
Injection-site reactions (pain, swelling, and erythema) and oral temperature were solicited using VRC-aided surveillance for five days after each injection of GARDASIL 9 during the clinical studies. The rates and severity of these solicited adverse reactions that occurred within five days following each dose of GARDASIL 9 compared with GARDASIL in Study 1 (girls and women 16 through 26 years of age) and Study 3 (girls 9 through 15 years of age) are presented in Table 1. Among subjects who received GARDASIL 9, the rates of injection-site pain were approximately equal across the three reporting time periods. Rates of injection-site swelling and injection-site erythema increased following each successive dose of GARDASIL 9. Recipients of GARDASIL 9 had numerically higher rates of injection-site reactions compared with recipients of GARDASIL.
Table 1: Rates (%) and Severity of Solicited Injection-Site and Systemic Adverse Reactions Occurring within Five Days of Each Vaccination with GARDASIL 9 Compared with GARDASIL (Studies 1 and 3)
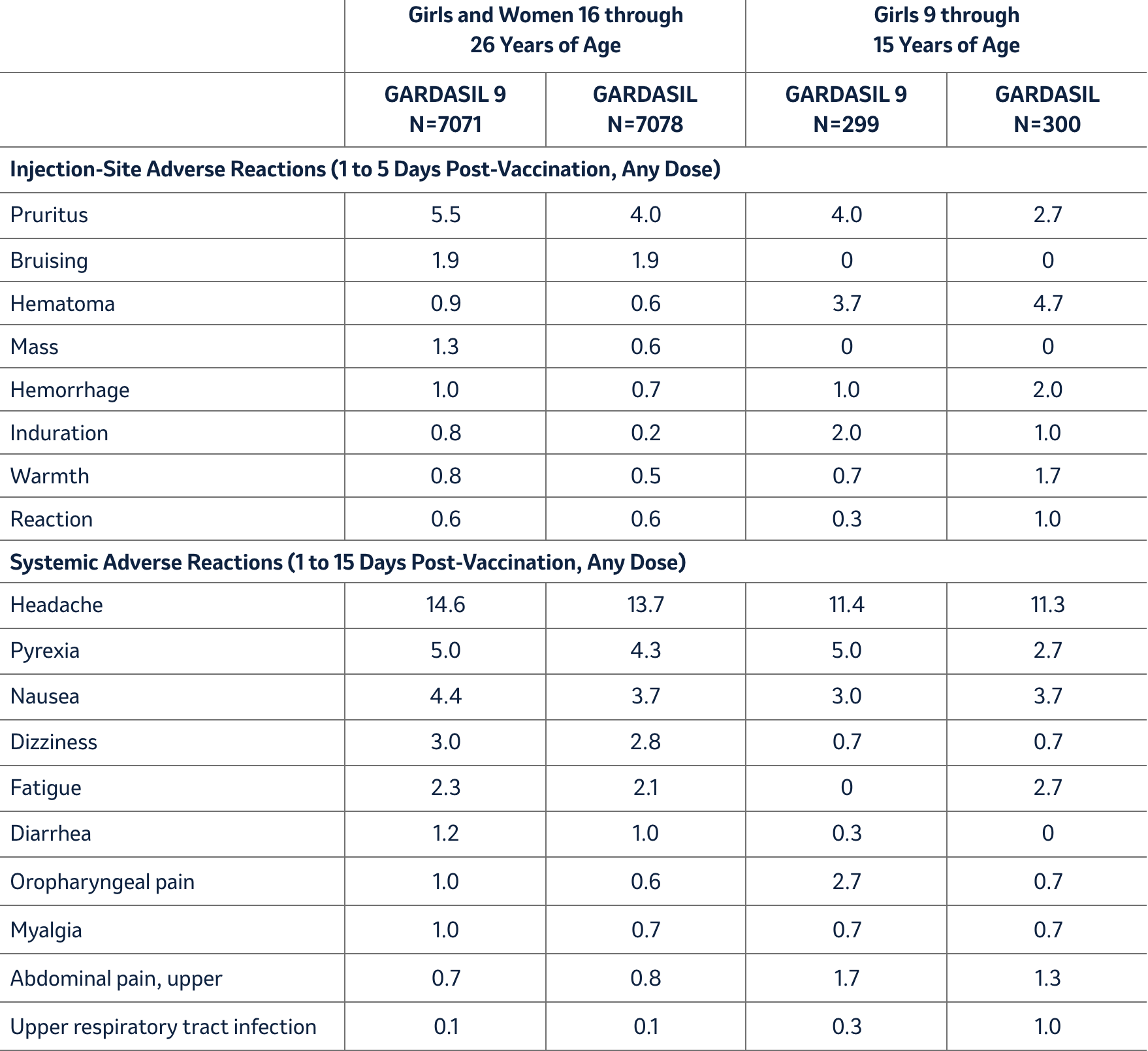
Unsolicited injection-site and systemic adverse reactions (assessed as vaccine-related by the investigator) observed among recipients of either GARDASIL 9 or GARDASIL in Studies 1 and 3 at a frequency of at least 1% are shown in Table 2. Few individuals discontinued study participation due to adverse experiences after receiving either vaccine (GARDASIL 9 = 0.1% vs. GARDASIL <0.1%).
Table 2: Rates (%) of Unsolicited Injection-Site and Systemic Adverse Reactions Occurring among ≥1.0% of Individuals after Any Vaccination with GARDASIL 9 Compared with GARDASIL (Studies 1 and 3)
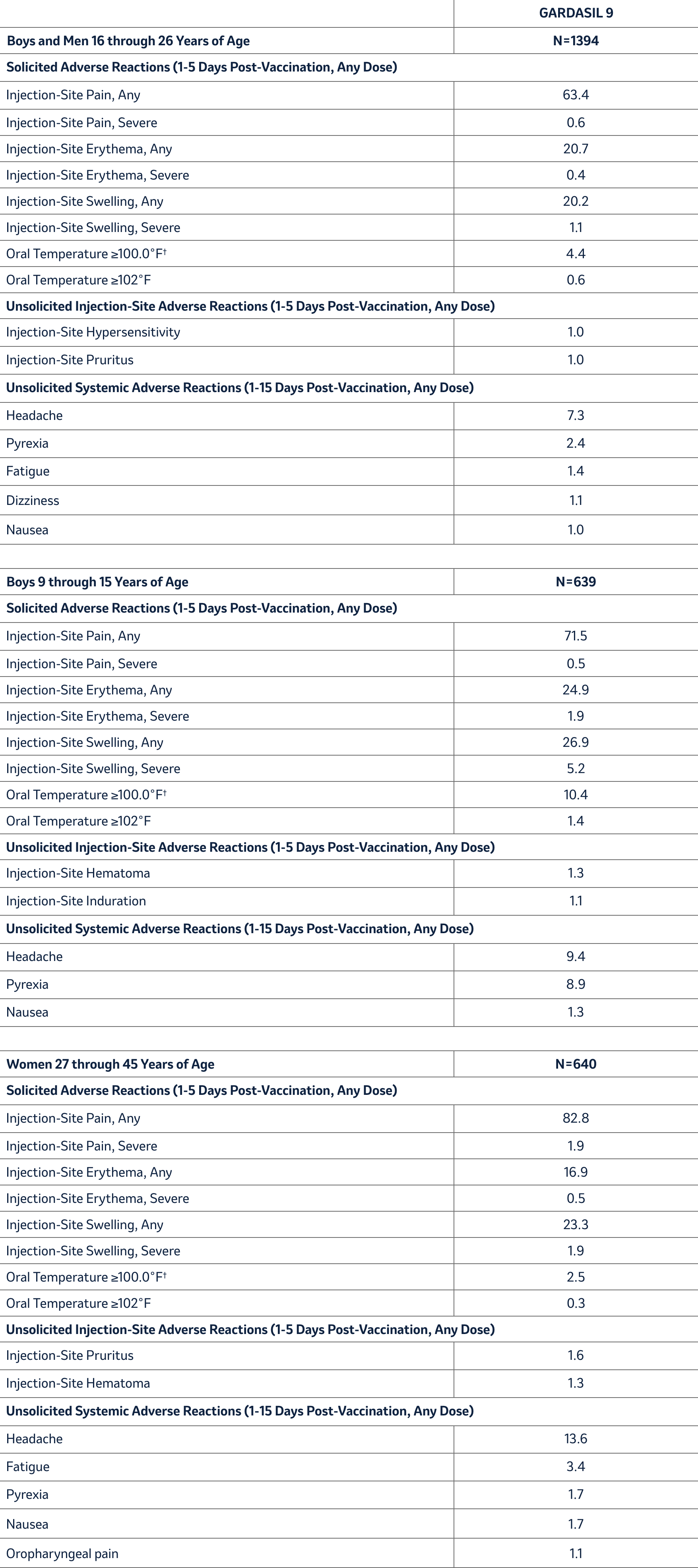
In an uncontrolled clinical trial with 639 boys and 1,878 girls 9 through 15 years of age (Study 2), the rates and severity of solicited adverse reactions following each dose of GARDASIL 9 were similar between boys and girls. Rates of solicited and unsolicited injection-site and systemic adverse reactions in boys 9 through 15 years of age were similar to those among girls 9 through 15 years of age. Solicited and unsolicited adverse reactions reported by boys in this study are shown in Table 3.
In another uncontrolled clinical trial with 1,394 boys and men and 1,075 girls and women 16 through 26 years of age (Study 7), the rates of solicited and unsolicited adverse reactions following each dose of GARDASIL 9 among girls and women 16 through 26 years of age were similar to those reported in Study 1. Rates of solicited and unsolicited adverse reactions reported by boys and men 16 through 26 years of age in this study are shown in Table 3.
In an uncontrolled clinical trial with 640 women 27 through 45 years of age and 570 girls and women 16 through 26 years of age (Study 9), the rates of solicited and unsolicited adverse reactions following each dose of GARDASIL 9 among girls and women 16 through 26 years of age were similar to those reported in Study 1. Rates of solicited and unsolicited adverse reactions reported by women 27 through 45 years of age in this study are shown in Table 3.
Table 3: Rates (%) of Solicited and Unsolicited* Injection-Site and Systemic Adverse Reactions among Boys 9 through 15 Years of Age, among Boys and Men 16 through 26 Years of Age and Women 27 through 45 Years of Age Who Received GARDASIL 9 (Studies 2, 7, and 9)
Serious Adverse Events in Clinical Studies
Serious adverse events were collected throughout the entire study period (range one month to 48 months post-last dose) for the seven clinical studies for GARDASIL 9. Out of the 15,705 individuals who were administered GARDASIL 9 and had safety follow-up, 354 reported a serious adverse event; representing 2.3% of the population. As a comparison, of the 7,378 individuals who were administered GARDASIL and had safety follow-up, 185 reported a serious adverse event; representing 2.5% of the population. Four GARDASIL 9 recipients each reported at least one serious adverse event that was determined to be vaccine-related. The vaccine-related serious adverse reactions were pyrexia, allergy to vaccine, asthmatic crisis, and headache
Deaths in the Entire Study Population
Across the clinical studies, ten deaths occurred (five each in the GARDASIL 9 and GARDASIL groups); none were assessed as vaccine-related. Causes of death in the GARDASIL 9 group included one automobile accident, one suicide, one case of acute lymphocytic leukemia, one case of hypovolemic septic shock, and one unexplained sudden death 678 days following the last dose of GARDASIL 9. Causes of death in the GARDASIL control group included one automobile accident, one airplane crash, one cerebral hemorrhage, one gunshot wound, and one stomach adenocarcinoma.
Systemic Autoimmune Disorders
In all of the clinical trials with GARDASIL 9 subjects were evaluated for new medical conditions potentially indicative of a systemic autoimmune disorder. In total, 2.2% (351/15,703) of GARDASIL 9 recipients and 3.3% (240/7,378) of GARDASIL recipients reported new medical conditions potentially indicative of systemic autoimmune disorders, which were similar to rates reported following GARDASIL, AAHS control, or saline placebo in historical clinical trials
Clinical Trials Experience for GARDASIL 9 in Individuals Who Have Been Previously Vaccinated with GARDASIL
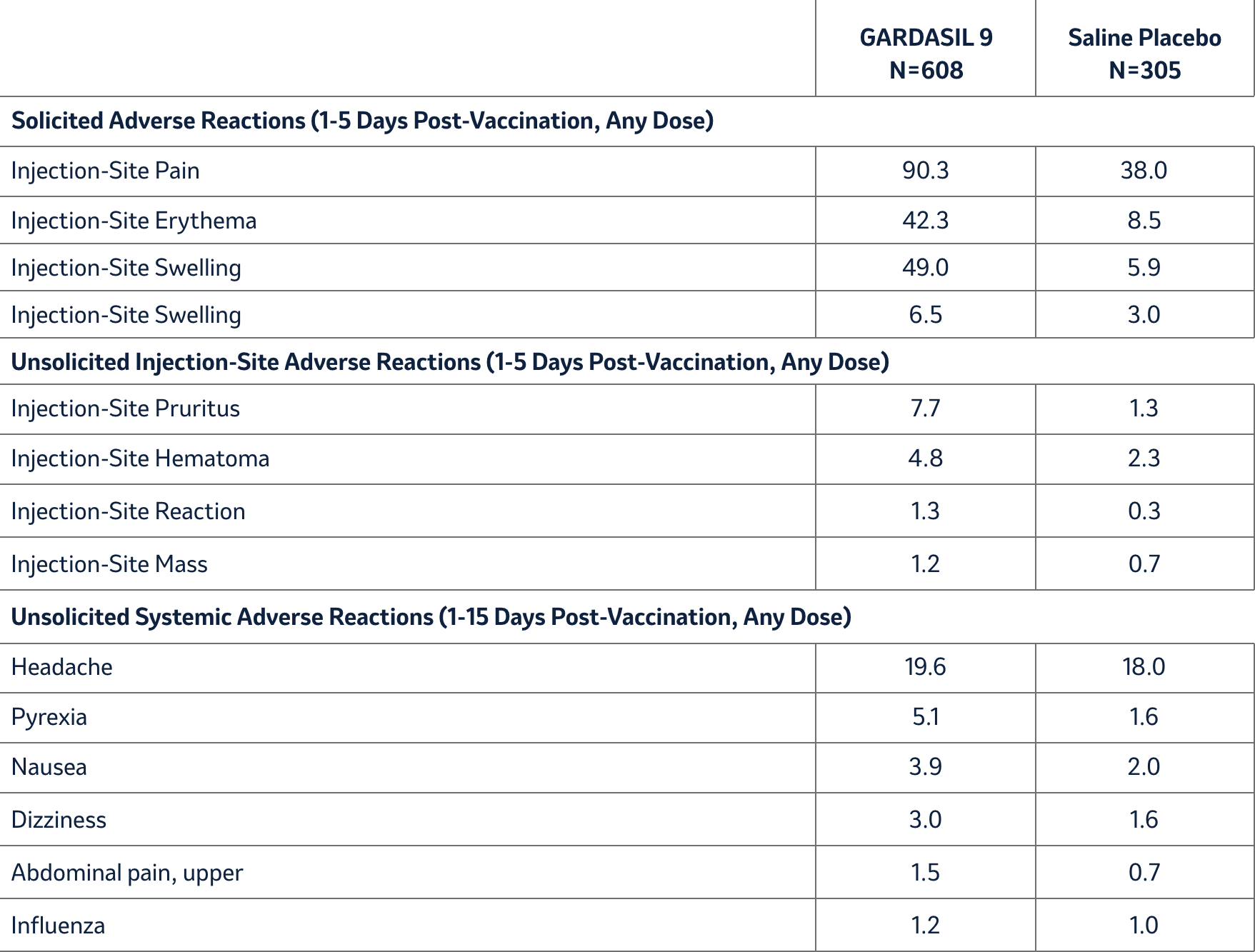
A clinical study (Study 4) evaluated the safety of GARDASIL 9 in 12- through 26-year-old girls and women who had previously been vaccinated with three doses of GARDASIL. The time interval between the last injection of GARDASIL and the first injection of GARDASIL 9 ranged from approximately 12 to 36 months. Individuals were administered GARDASIL 9 or saline placebo and safety was evaluated using VRC-aided surveillance for 14 days after each injection of GARDASIL 9 or saline placebo in these individuals. The individuals who were monitored included 608 individuals who received GARDASIL 9 and 305 individuals who received saline placebo. Few (0.5%) individuals who received GARDASIL 9 discontinued due to adverse reactions. The vaccine-related adverse experiences that were observed among recipients of GARDASIL 9 at a frequency of at least 1.0% and also at a greater frequency than that observed among saline placebo recipients are shown in Table 4. Overall, the safety profile was similar between individuals vaccinated with GARDASIL 9 who were previously vaccinated with GARDASIL and those who were naïve to HPV vaccination with the exception of numerically higher rates of injection-site swelling and erythema among individuals who were previously vaccinated with GARDASIL (Tables 1 and 4).
Table 4: Rates (%) of Solicited and Unsolicited* Injection-Site and Systemic Adverse Reactions among Individuals Previously Vaccinated with GARDASIL Who Received GARDASIL 9 or Saline Placebo (Girls and Women 12 through 26 Years of Age) (Study 4)
Safety in Concomitant Use with Menactra and Adacel
In Study 5, the safety of GARDASIL 9 when administered concomitantly with Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap)] was evaluated in a randomized study of 1,241 boys (n = 620) and girls (n = 621) with a mean age of 12.2 years.
Of the 1,237 boys and girls vaccinated, 1,220 had safety follow-up for injection-site adverse reactions. The rates of injection-site adverse reactions were similar between the concomitant group and non-concomitant group (vaccination with GARDASIL 9 separated from vaccination with Menactra and Adacel by 1 month) with the exception of an increased rate of swelling reported at the injection site for GARDASIL 9 in the concomitant group (14.4%) compared to the non-concomitant group (9.4%). The majority of injection-site swelling adverse reactions were reported as being mild to moderate in intensity.
Postmarketing Experience
The postmarketing adverse experiences were reported voluntarily from a population of uncertain size, therefore, it is not possible to reliably estimate their frequency or to establish a causal relationship to vaccine exposure.
The safety profile of GARDASIL 9 and GARDASIL are similar. The postmarketing safety experience with GARDASIL is relevant to GARDASIL 9 since the vaccines are manufactured similarly and contain the same L1 HPV proteins of four of the same HPV types.
GARDASIL 9
In addition to the adverse reactions reported in the clinical studies, the following adverse experiences have been spontaneously reported during post-approval use of GARDASIL 9:
Gastrointestinal disorders: Vomiting
Skin and subcutaneous tissue disorders: Urticaria
GARDASIL
Additionally, the following postmarketing adverse experiences have been spontaneously reported for GARDASIL:
Blood and lymphatic system disorders: Autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, lymphadenopathy.
Respiratory, thoracic and mediastinal disorders: Pulmonary embolus.
Gastrointestinal disorders: Pancreatitis.
General disorders and administration site conditions: Asthenia, chills, death, malaise.
Immune system disorders: Autoimmune diseases, hypersensitivity reactions including anaphylactic/anaphylactoid reactions, bronchospasm.
Musculoskeletal and connective tissue disorders: Arthralgia, myalgia.
Nervous system disorders: Acute disseminated encephalomyelitis, Guillain-Barré syndrome, motor neuron disease, paralysis, seizures, transverse myelitis.
Infections and infestations: Cellulitis.
Vascular disorders: Deep venous thrombosis
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. There are no adequate and well-controlled studies of GARDASIL 9 in pregnant women. Data from GARDASIL are relevant to GARDASIL 9 because both vaccines are manufactured using the same process and have overlapping compositions. Data were evaluated from pre-licensure studies in which 55 and 62 pregnancies with known outcomes were associated with women inadvertently exposed to GARDASIL and GARDASIL 9, respectively. Pregnancy Exposure Registry data are available from 1640 and 69 prospectively enrolled women with known pregnancy outcomes who were vaccinated with GARDASIL and GARDASIL 9, respectively. Available data do not suggest an increased risk of major birth defects and miscarriage in women who received GARDASIL or GARDASIL 9. In one developmental toxicity study, 0.5 mL of a vaccine formulation containing between 1 and 1.5 –fold of each of the 9 HPV antigen types was administered to female rats prior to mating and during gestation. In a second study, animals were administered a single human dose (0.5 mL) of GARDASIL 9 prior to mating, during gestation and during lactation. These animal studies revealed no evidence of harm to the fetus due to GARDASIL 9
Lactation
Risk summary
Available data are not sufficient to assess the effects of GARDASIL 9 on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GARDASIL 9 and any potential adverse effects on the breastfed child from GARDASIL 9 or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients below 9 years of age.
Geriatric Use
The safety and effectiveness of GARDASIL 9 have not been evaluated in a geriatric population, defined as individuals aged 65 years and over.
Immunocompromised Individuals
The immunologic response to GARDASIL 9 may be diminished in immunocompromised individuals.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
GARDASIL 9 has not been evaluated for the potential to cause carcinogenicity, genotoxicity or impairment of male fertility
For additional safety information please refer to Gardasil™9 Prescribing Information.
KW-GSL-00014 | 24/02/2027
