
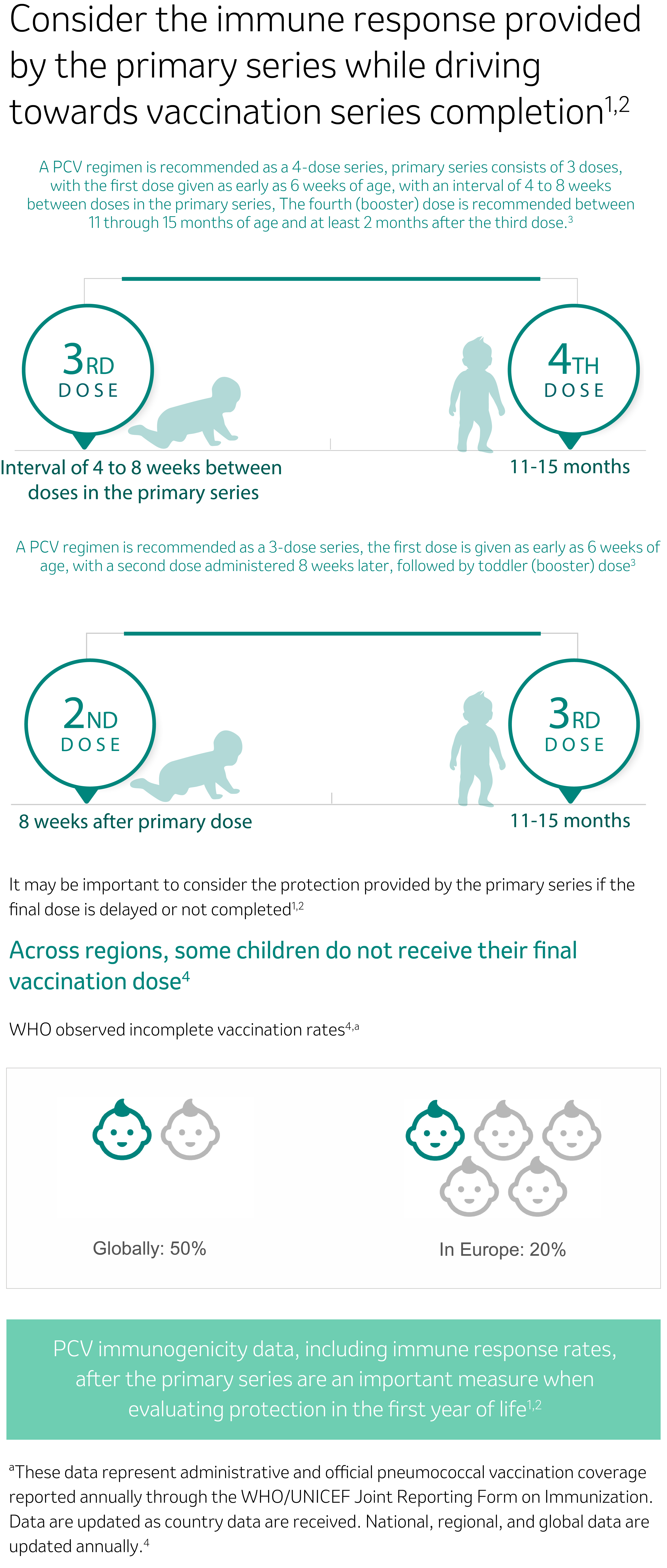

PCV, pneumococcal vaccine conjugate; WHO, World Health Organization; UNICEF, The United Nations Children’s Fund.
References:
-
Guidelines on clinical evaluation of vaccines: regulatory expectations. WHO Technical Report Series 1004, Annex 9, 2017. World Health Organization. Accessed February 6, 2025.
https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9. -
Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines, Annex 3, TRS No 977. World Health Organization. October 19,
Accessed February 6, 2025. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977. - Vaxneuvance summary of product characteristics.
- World Health Organization. Pneumococcal vaccination coverage. Accessed February 6, 2025. https://immunizationdata.who.int/pages/coverage/pcv.html?CODE=Global+EUR+DE
SA-NON-00431 | Exp 5 Feb 2027