KN 426 ORR


References:
- Keytruda Summary of Product Characteristics.
- Kantar Health. Treatment Architecture: Renal Cell Carcinoma.Cancer MPact®. EU5. 2020;1–89.
AE-RCC-00004 | Exp : 06 JUN 2024
AE-RCC-00003 | Exp : 06 JUN 2024
QA-RCC-00001 | Exp : 06 JUN 2024
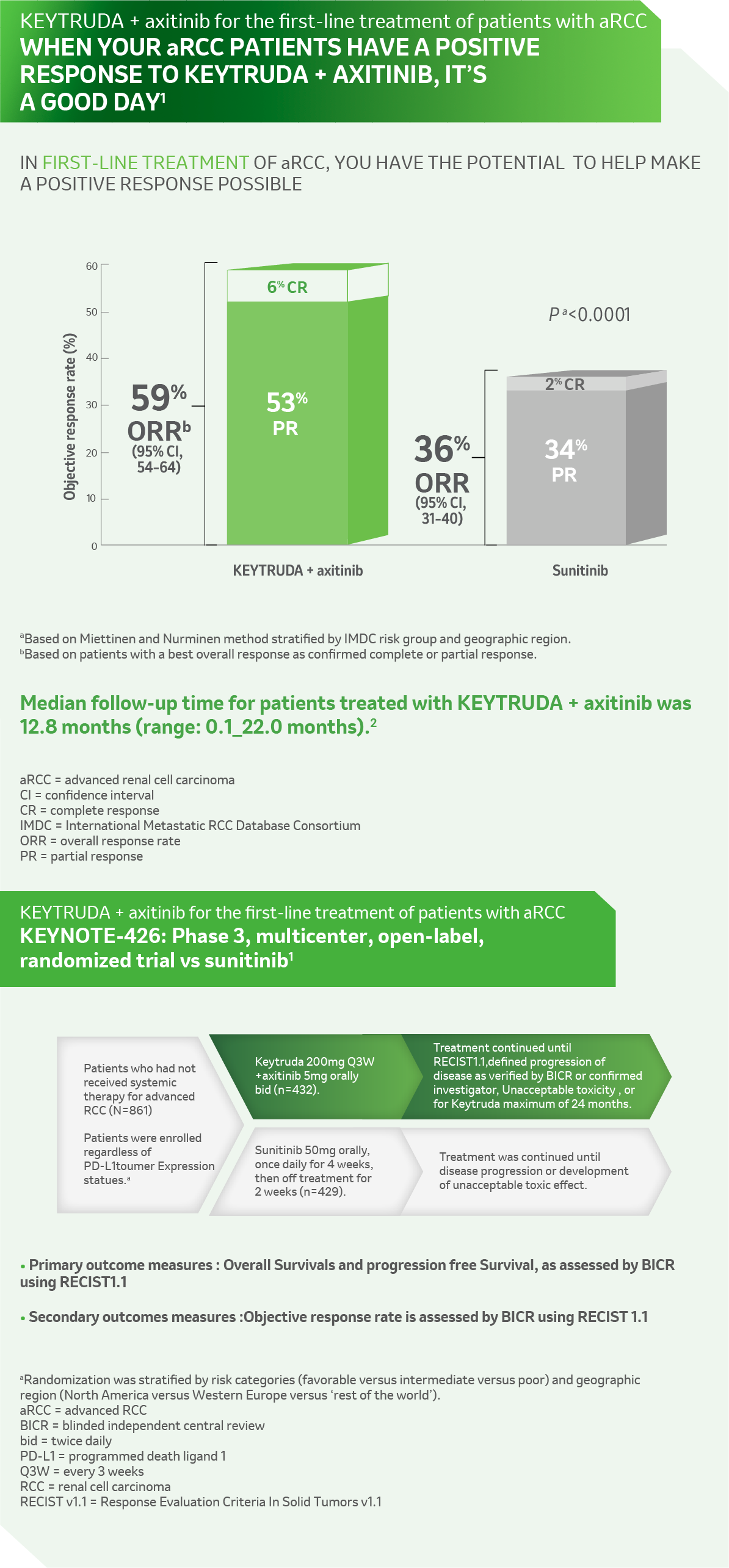
References:
- Keytruda Summary of Product Characteristics.
- Rini BI, Plimack ER, Stus V, et al; for the KEYNOTE-426 investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019; 380 (12): 1116–1127.
AE-RCC-00004 | Exp : 06 JUN 2024
AE-RCC-00003 | Exp : 06 JUN 2024
QA-RCC-00001 | Exp : 06 JUN 2024

References:
- Keytruda Summary of Product Characteristics.
- ASCO 2023: Pembrolizumab (Pembro) plus axitinib (AXI) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccrcc): Results of 5 years follow-up of keynote-426 (no date) UroToday. Available at: https://www.urotoday.com/conference-highlights/asco-2023/asco-2023-kidney-cancer/144965-asco-2023-pembrolizumab-axitinib-versus-sunitinib-as-first-line-therapy-for-advanced-clear-cell-rcc-5-year-analysis-of-keynote-426.html (Accessed: 20 August 2023).
AE-RCC-00004 | Exp : 06 JUN 2024
AE-RCC-00003 | Exp : 06 JUN 2024
QA-RCC-00001 | Exp : 06 JUN 2024
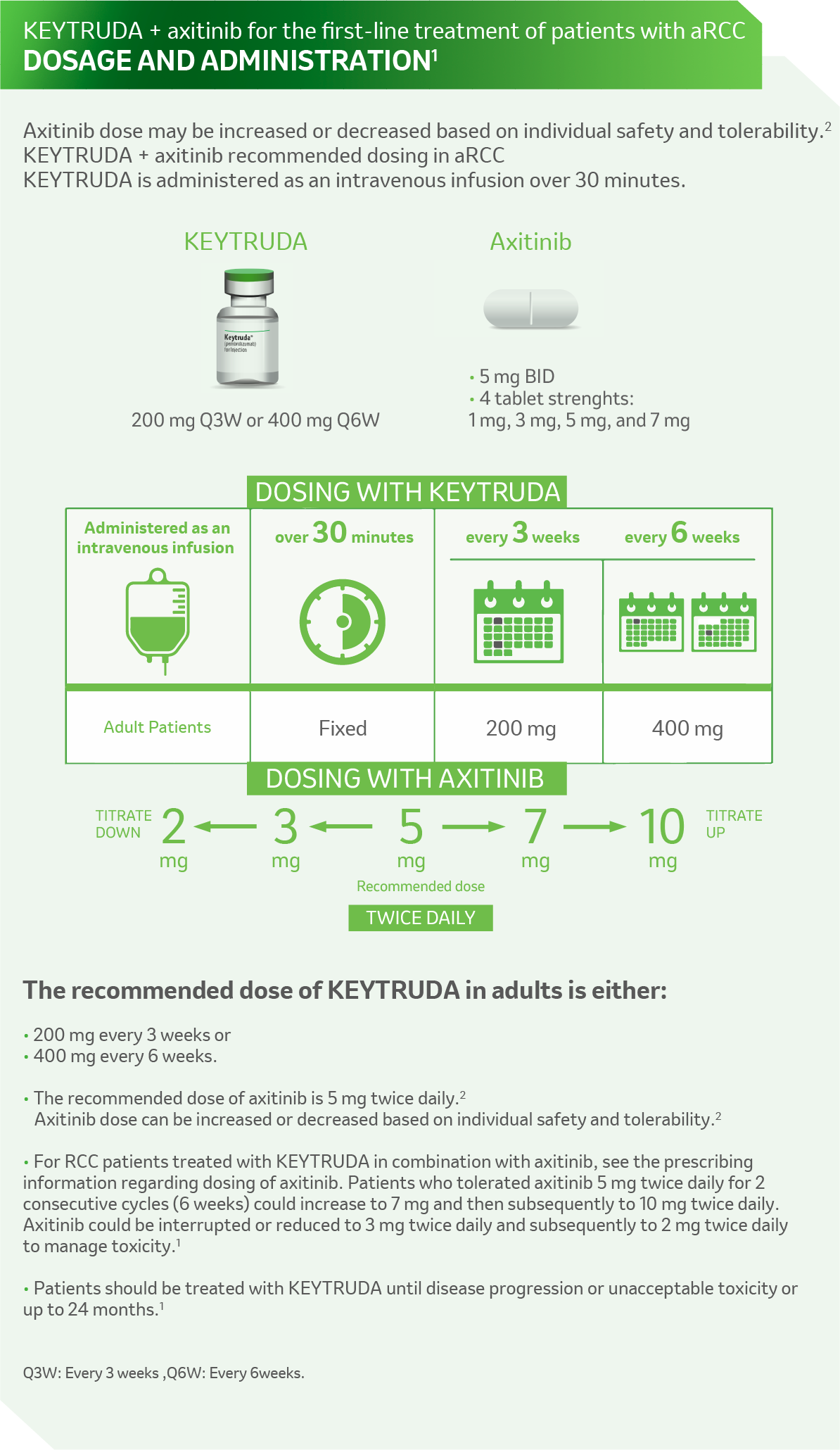
References:
- Keytruda Summary of Product Characteristics.
- Inlyta [SmPC]. Belgium: Pfizer Europe MA EEIG; 2021
AE-RCC-00004 | Exp : 06 JUN 2024
AE-RCC-00003 | Exp : 06 JUN 2024
QA-RCC-00001 | Exp : 06 JUN 2024
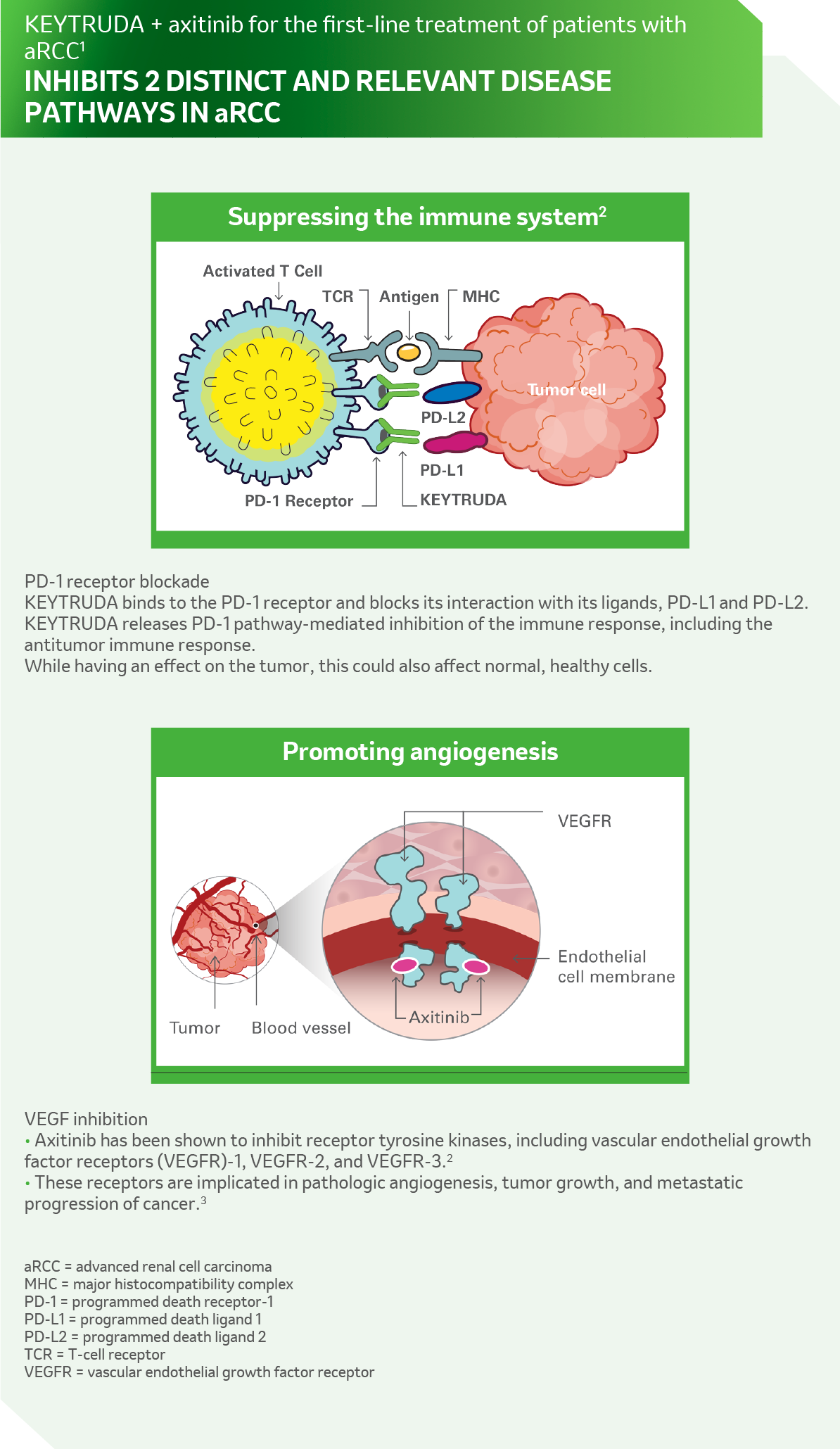
References:
- Keytruda Summary of Product Characteristics.
- Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4),252–264.
https://doi.org/10.1038/nrc3239. - Inlyta [SmPC]. Belgium: Pfizer Europe MA EEIG; 2021
AE-RCC-00004 | Exp : 06 JUN 2024
AE-RCC-00003 | Exp : 06 JUN 2024
QA-RCC-00001 | Exp : 06 JUN 2024

References:
- Keytruda Summary of Product Characteristics.
- Ljungberg, B., Albiges, L., Abu-Ghanem, Y., Bedke, J., Capitanio, U., Dabestani, S. (2022). European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. European Urology, 82(4 ), 399-410.
https://doi.org/10.1016/j.eururo.2022.03.006
AE-RCC-00004 | Exp : 06 JUN 2024
AE-RCC-00003 | Exp : 06 JUN 2024
QA-RCC-00001 | Exp : 06 JUN 2024