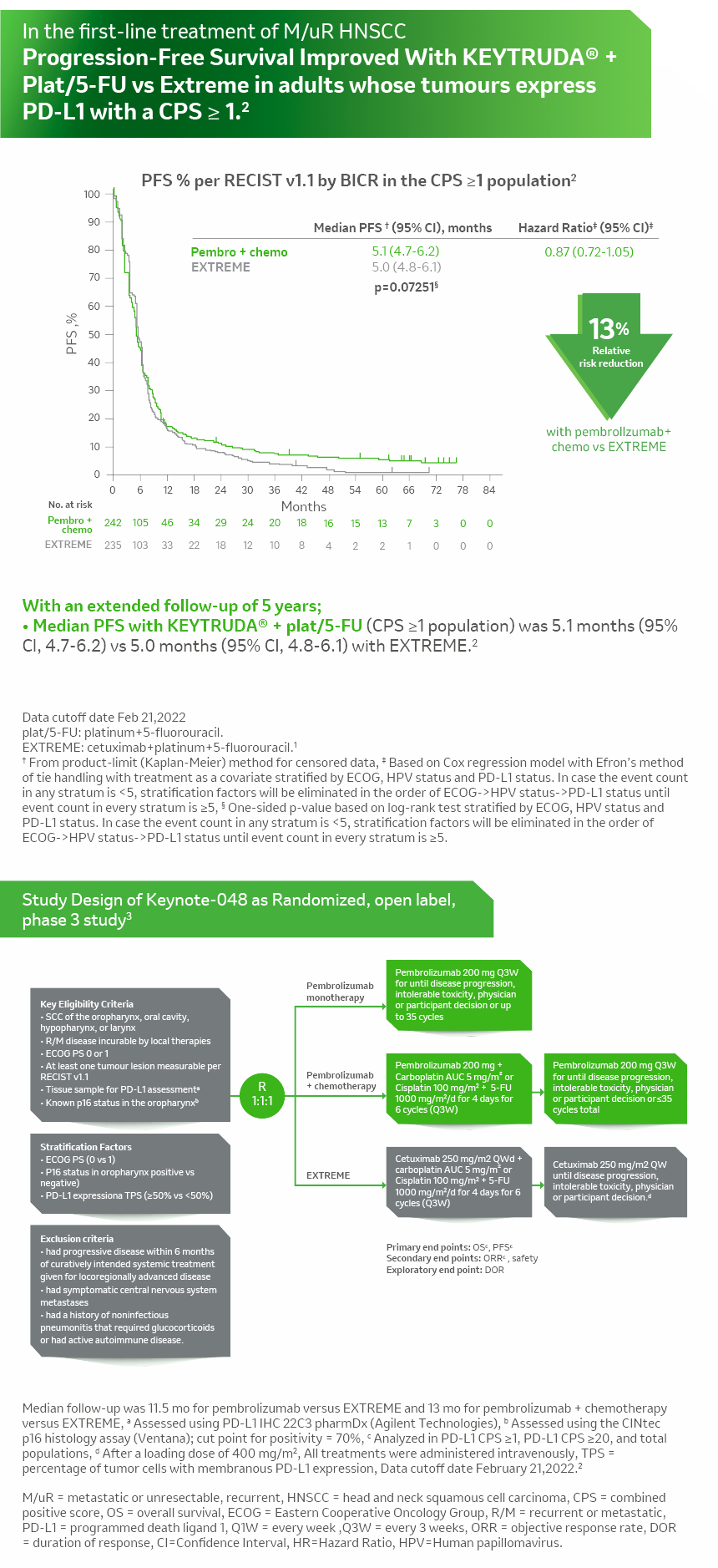
KN- 048 Combination esmo


References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10212):1915-1928.
References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10212):1915-1928.

References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10212):1915-1928.
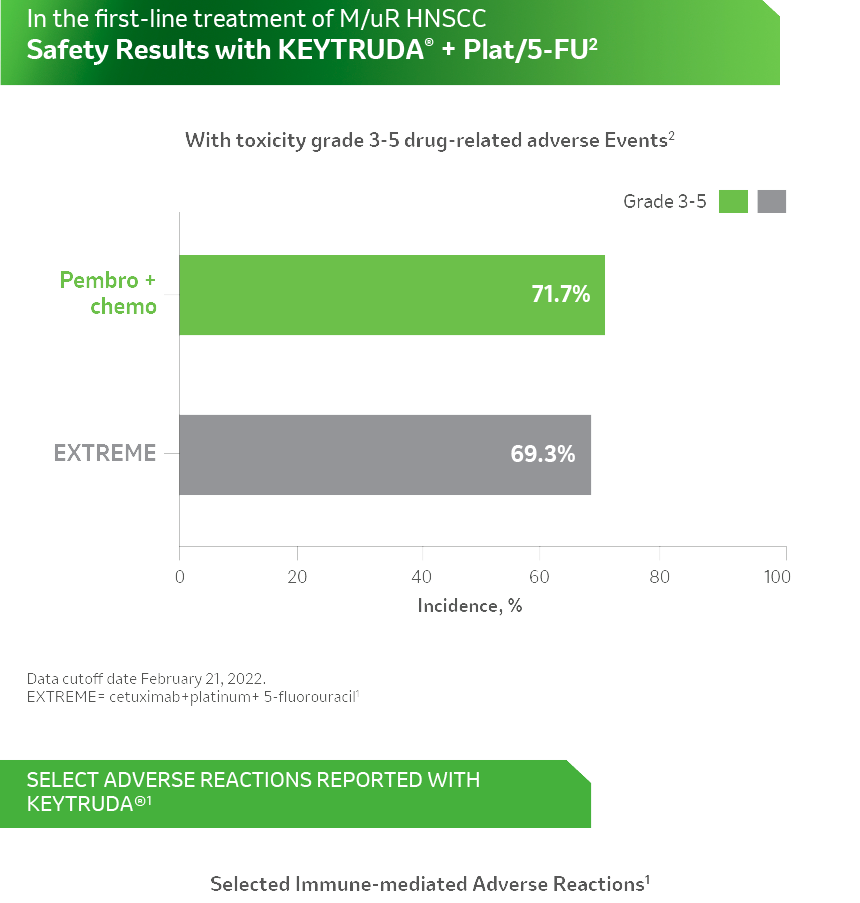
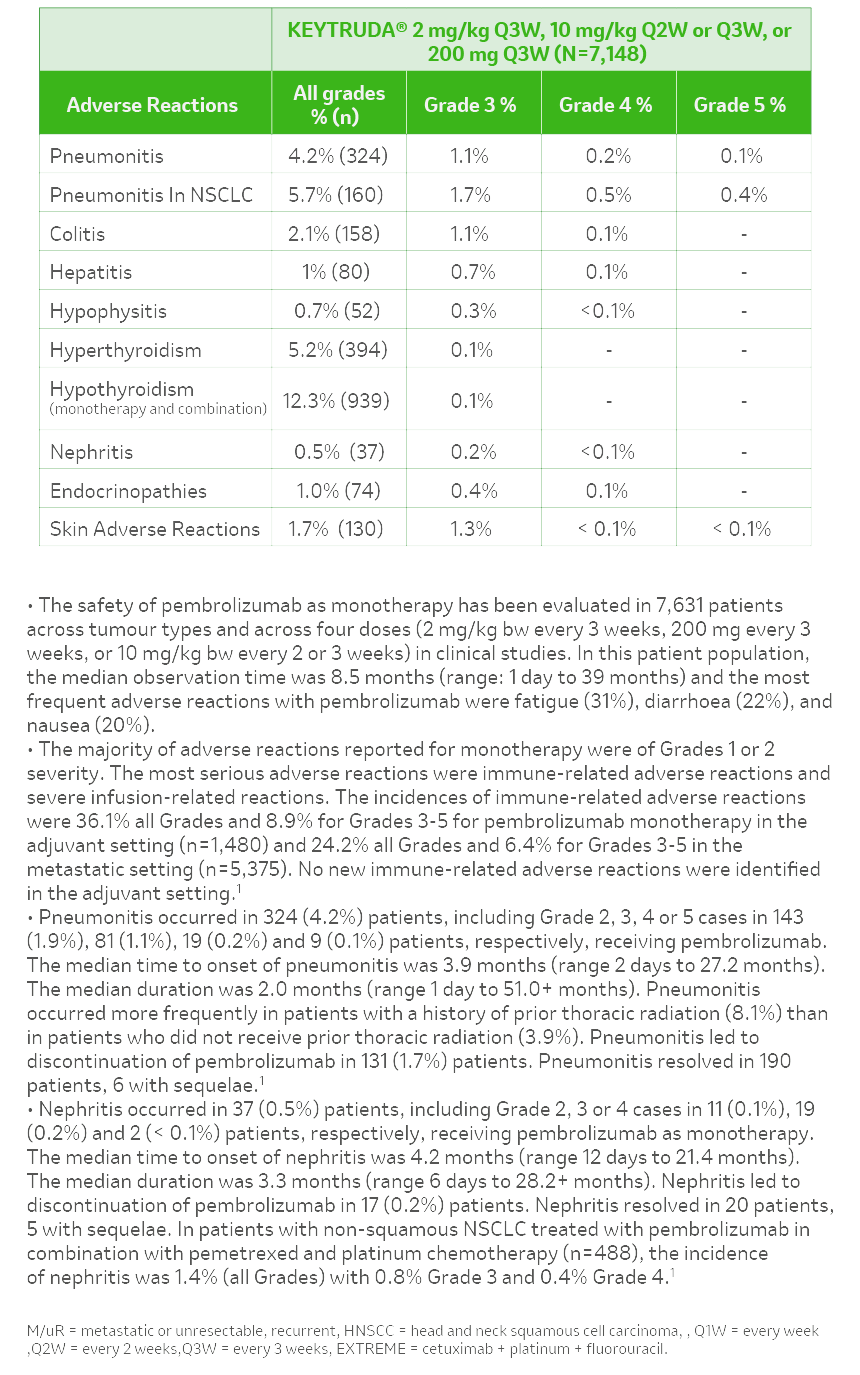
References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
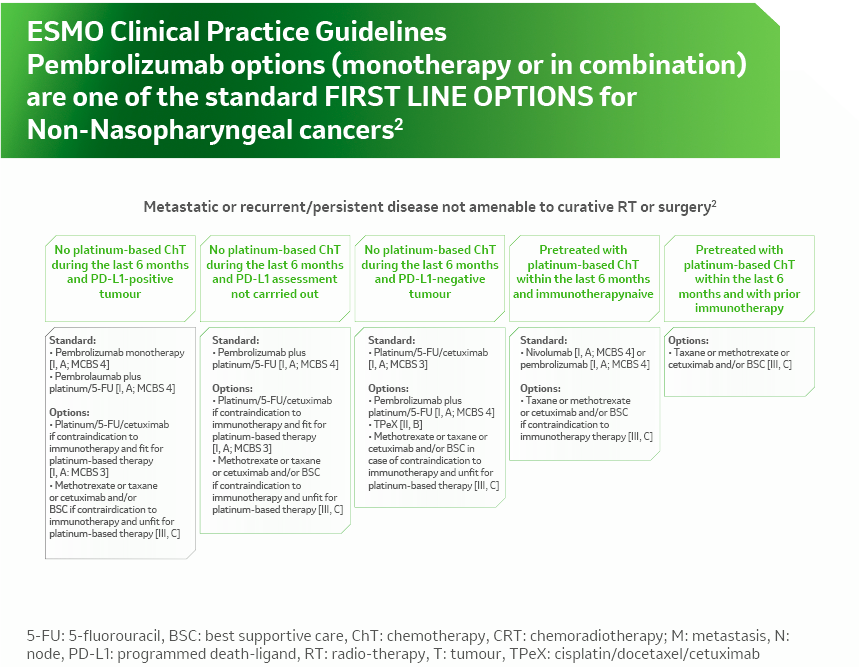
References:
- Keytruda® SPC.
- Machiels JP, René Leemans C, Golusinski W, et al: Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol.2020 ;31(11):1462-1475.
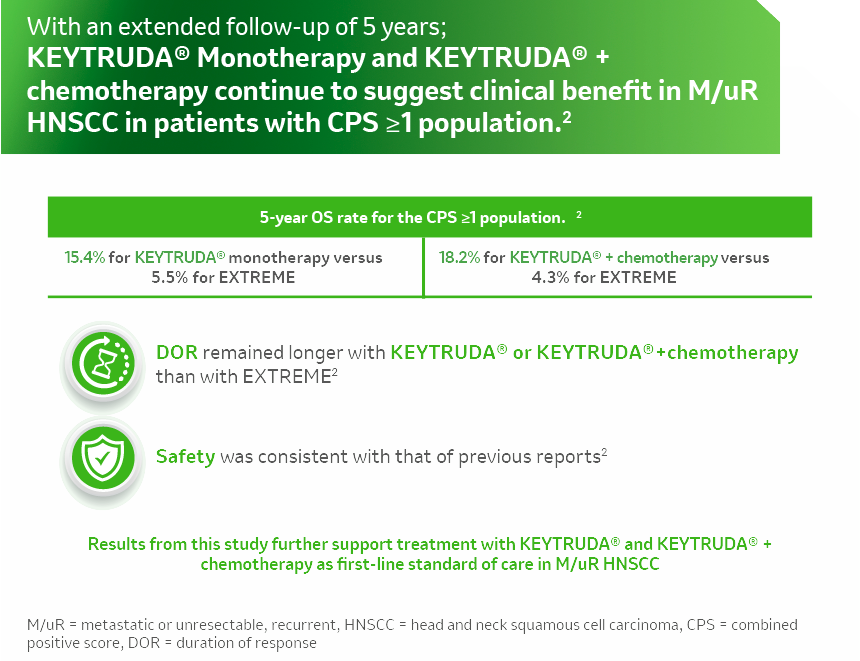
References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.B
SA-OHN-00027 | Exp: 30 Dec 2024.