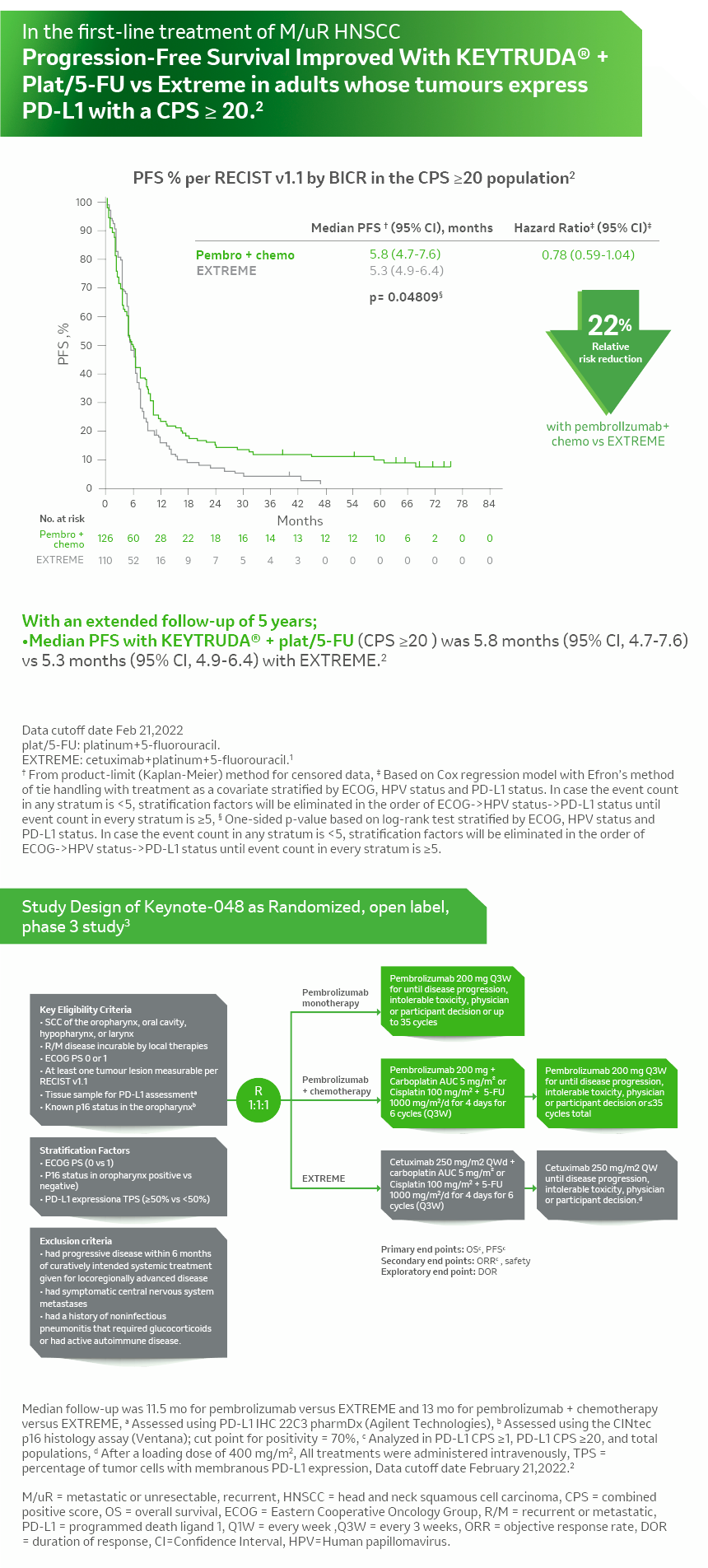
KN- 048 Combination PFS


References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10212):1915-1928.
References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10212):1915-1928.

References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 ;394(10212):1915-1928.
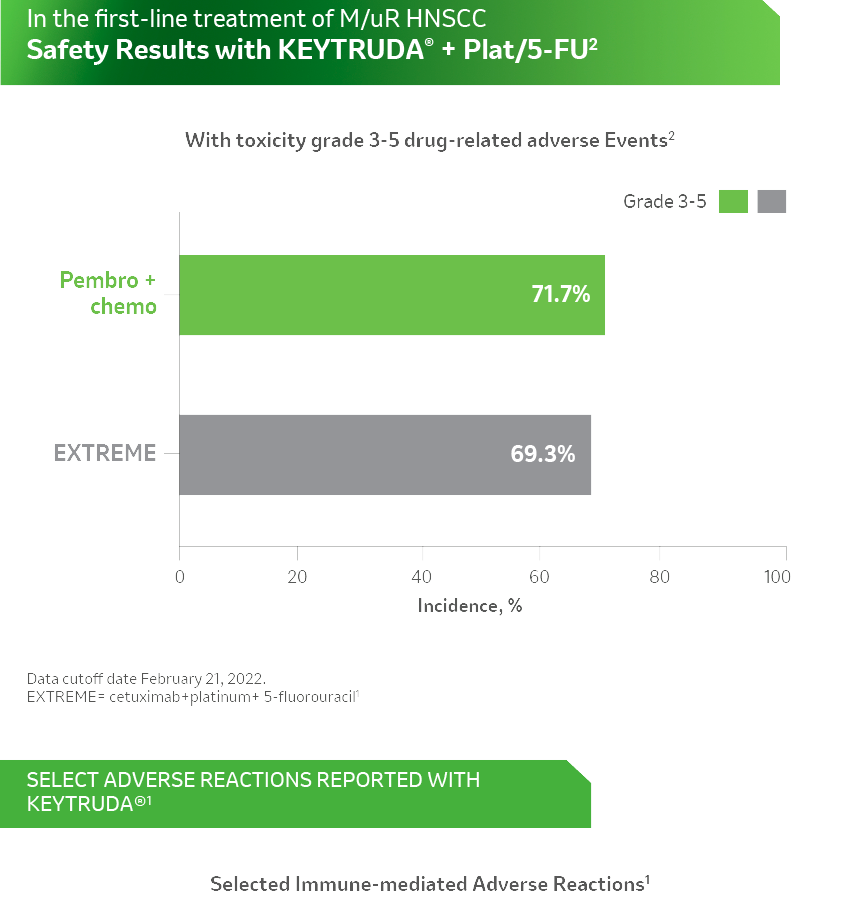
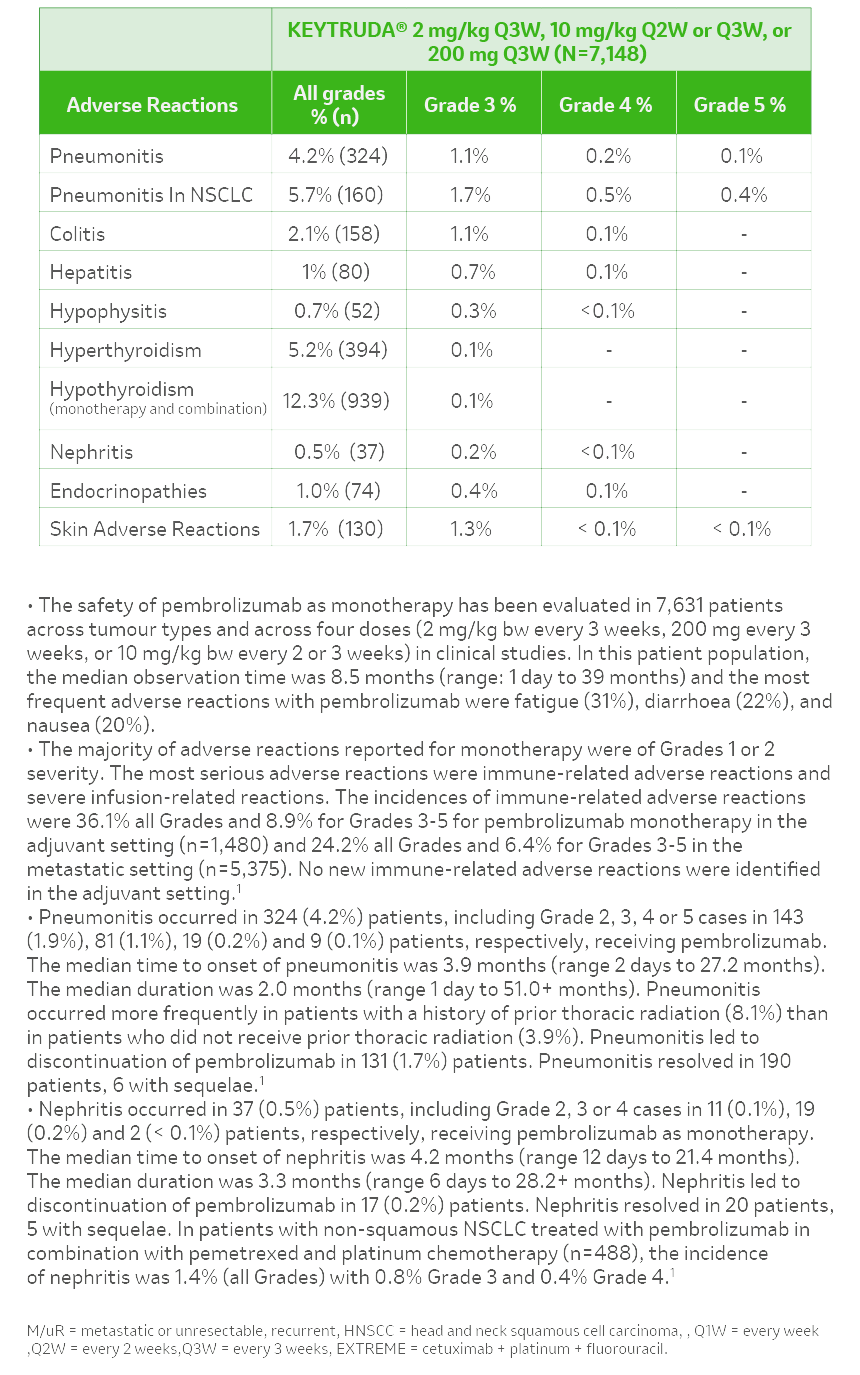
References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.B

References:
- Keytruda® SPC.
- National Comprehensive Cancer Network. NCCN Guidelines. Version 2.2024. Head and Neck Cancers. Available at:https://www.nccn.org/guidelines/guidelinesprocess/transparency-process-and-recommendations/GetFileFromFile ManagerGuid?FileManagerGuidId=c4edff60-3e93-45d9-8503-5892d71ea8be. Last accessed: 22/4/2024. All rights reserved. content are trademarks owned by the National Comprehensive cancer Network
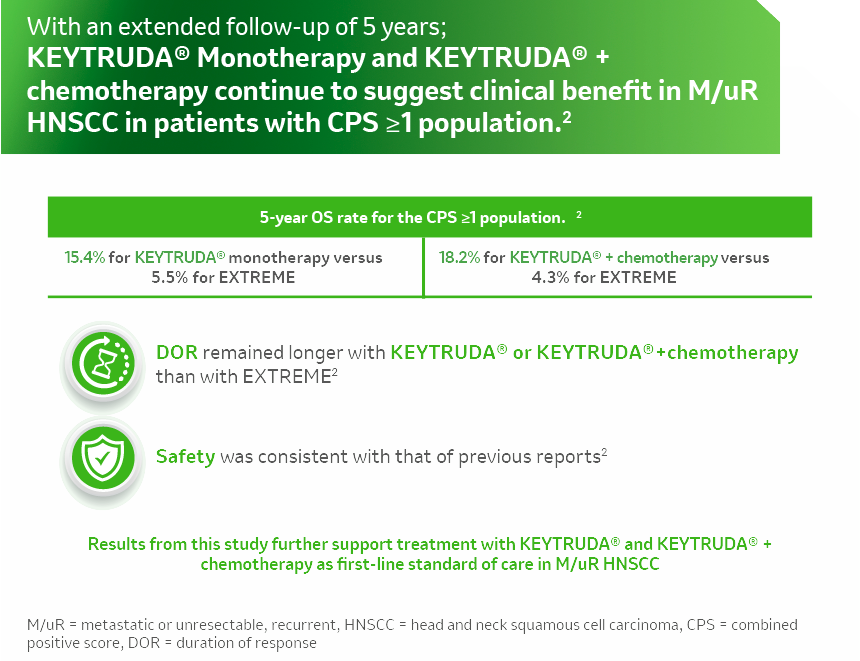
References:
- Keytruda® SPC.
- MSD DATA ON FILE KN048- 5-YEAR POSTHOC ANALYSIS SEPTEMBER 28,2022.B
SA-OHN-00027 | Exp: 30 Dec 2024.